
 Copy to clipboard
Copy to clipboard 
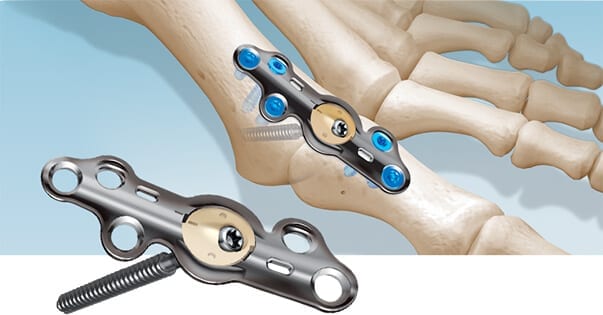
In2Bones USA entered into an exclusive agreement with Invibio to collaborate on research, development and manufacturing that will apply Invibio’s PEEK-OPTIMA™ Ultra Reinforced (POUR) carbon fiber technology to In2Bones’ lower extremity implants, like CoLink® View Plates pictured above.
Both companies will make significant investments and share technical capabilities, intellectual property, marketing, clinical data and labor. First products arising from the agreement are slated for 2021. In2Bones intends to incorporate the POUR technology in additional implant designs.
POUR technology combines the mechanical and thermal properties of PEEK-OPTIMA natural polymers, including radiolucency for artifact-free imaging, alongside the strength of continuous carbon fiber. The biomaterial’s modulus of elasticity is closer to that of bone than metal to promote faster healing.
Alan Taylor, President and CEO of In2Bones Global, said, “Most orthopedic fixation plates are manufactured from metal which often blocks and distorts x-rays. The POUR biomaterial allows the surgeon to see through the plate via x-ray and verify healing.”
John Devine, Medical Business Director, Invibio Biomaterial Solutions, said, “We have enjoyed watching In2Bones’ rapid growth and innovation across its extremity product line over the past few years and are excited to collaborate with the company to realize the potential of POUR technology — and develop the fixation devices of the future.”
In2Bones USA entered into an exclusive agreement with Invibio to collaborate on research, development and manufacturing that will apply Invibio’s PEEK-OPTIMA™ Ultra Reinforced (POUR) carbon fiber technology to In2Bones’ lower extremity implants, like CoLink® View Plates pictured above.
Both companies will make significant investments and...
In2Bones USA entered into an exclusive agreement with Invibio to collaborate on research, development and manufacturing that will apply Invibio’s PEEK-OPTIMA™ Ultra Reinforced (POUR) carbon fiber technology to In2Bones’ lower extremity implants, like CoLink® View Plates pictured above.
Both companies will make significant investments and share technical capabilities, intellectual property, marketing, clinical data and labor. First products arising from the agreement are slated for 2021. In2Bones intends to incorporate the POUR technology in additional implant designs.
POUR technology combines the mechanical and thermal properties of PEEK-OPTIMA natural polymers, including radiolucency for artifact-free imaging, alongside the strength of continuous carbon fiber. The biomaterial’s modulus of elasticity is closer to that of bone than metal to promote faster healing.
Alan Taylor, President and CEO of In2Bones Global, said, “Most orthopedic fixation plates are manufactured from metal which often blocks and distorts x-rays. The POUR biomaterial allows the surgeon to see through the plate via x-ray and verify healing.”
John Devine, Medical Business Director, Invibio Biomaterial Solutions, said, “We have enjoyed watching In2Bones’ rapid growth and innovation across its extremity product line over the past few years and are excited to collaborate with the company to realize the potential of POUR technology — and develop the fixation devices of the future.”

You are out of free articles for this month
Subscribe as a Guest for $0 and unlock a total of 5 articles per month.
You are out of five articles for this month
Subscribe as an Executive Member for access to unlimited articles, THE ORTHOPAEDIC INDUSTRY ANNUAL REPORT and more.
JV
Julie Vetalice is ORTHOWORLD's Editorial Assistant. She has covered the orthopedic industry for over 20 years, having joined the company in 1999.