
 Copy to clipboard
Copy to clipboard 
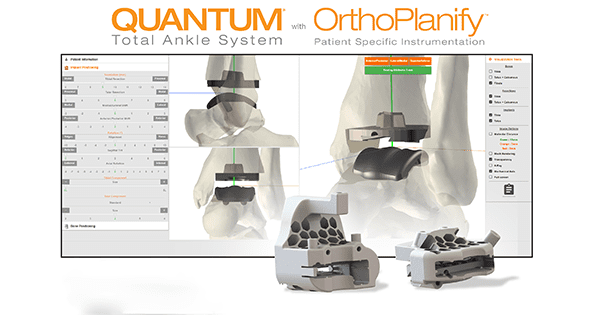
In2Bones announced FDA clearance of OrthoPlanify™ patient-specific planning software and 3D-printed cutting guides, both part of the company’s QUANTUM®Total Ankle System.
In2Bones’ Orthoplanify software integrates patient CT scans and X-rays, enabling surgeons to easily modify, adjust and manipulate placement of the QUANTUM Total Ankle System implant prior to surgery.
During surgery, the Total Ankle System’s custom 3D-printed cutting guides save surgeons multiple steps and allow for precision bone cuts and more accurate implant alignment when compared with manual instrumentation.
QUANTUM is designed to provide implant stability due to its vertical, cruciate-shaped tibial stem. The talar component, available in flat and chamfer cut options, features an anatomically designed double radius of curvature. This combination is intended to improve function and longevity compared to currently available total ankle products.
Source: In2Bones Global
In2Bones announced FDA clearance of OrthoPlanify™ patient-specific planning software and 3D-printed cutting guides, both part of the company’s QUANTUM®Total Ankle System.
In2Bones’ Orthoplanify software integrates patient CT scans and X-rays, enabling surgeons to easily modify, adjust and manipulate placement of the QUANTUM Total Ankle System...
In2Bones announced FDA clearance of OrthoPlanify™ patient-specific planning software and 3D-printed cutting guides, both part of the company’s QUANTUM®Total Ankle System.
In2Bones’ Orthoplanify software integrates patient CT scans and X-rays, enabling surgeons to easily modify, adjust and manipulate placement of the QUANTUM Total Ankle System implant prior to surgery.
During surgery, the Total Ankle System’s custom 3D-printed cutting guides save surgeons multiple steps and allow for precision bone cuts and more accurate implant alignment when compared with manual instrumentation.
QUANTUM is designed to provide implant stability due to its vertical, cruciate-shaped tibial stem. The talar component, available in flat and chamfer cut options, features an anatomically designed double radius of curvature. This combination is intended to improve function and longevity compared to currently available total ankle products.
Source: In2Bones Global

You are out of free articles for this month
Subscribe as a Guest for $0 and unlock a total of 5 articles per month.
You are out of five articles for this month
Subscribe as an Executive Member for access to unlimited articles, THE ORTHOPAEDIC INDUSTRY ANNUAL REPORT and more.
JV
Julie Vetalice is ORTHOWORLD's Editorial Assistant. She has covered the orthopedic industry for over 20 years, having joined the company in 1999.







