
 Copy to clipboard
Copy to clipboard 
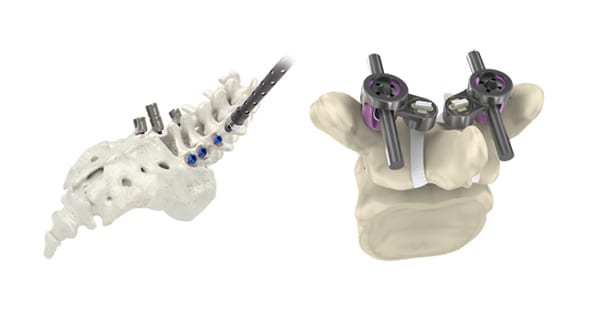
Implanet and SeaSpine were granted FDA 510(k) clearance to market the Mariner Cap System, a combination of SeaSpine’s Mariner® pedicle screw system with Implanet’s JAZZ Cap® technology (shown above, left to right).
Implanet and SeaSpine co-developed Mariner Cap to facilitate the treatment of adult degenerative disorders with novel stabilization of pedicle screws. Product launch plans are underway.
Implanet and SeaSpine have been in partnership since February 2019. SeaSpine holds exclusive access and distribution rights for products co-developed with the JAZZ range under its own brand name in the U.S.
Ludovic Lastennet, CEO of Implanet, said, “The FDA clearance of the Mariner Cap System by SeaSpine is a major milestone in this partnership initiated 18 months ago, and reflects the clinical value of our proprietary JAZZ® technology. This implant, henceforth adapted to the SeaSpine Mariner® system, will be deployed over the coming months and provides a direct response to the technological innovation requirements of the world’s largest market for the treatment of spinal disorders. This new system will enable SeaSpine to provide a distinctive and high-value-added offering in this market. The success of this collaboration with the materialization of the Mariner Cap System echoes that obtained by our JAZZ Cap® solution during the first surgeries performed in the United States in complex adult deformity indications.”
Keith Valentine, President and CEO of SeaSpine, said, “The Mariner Cap System further strengthens our range of products dedicated to treating spinal disorders, and represents a proprietary alternative that we can offer our distributors, surgeons and their patients across the US. Combining our two technologies provides a unique solution for securing the stability of pedicle screw constructs. We look forward to continuing our innovative partnership with Implanet.”
Implanet and SeaSpine were granted FDA 510(k) clearance to market the Mariner Cap System, a combination of SeaSpine’s Mariner® pedicle screw system with Implanet’s JAZZ Cap® technology (shown above, left to right).
Implanet and SeaSpine co-developed Mariner Cap to facilitate the treatment of adult degenerative disorders with novel...
Implanet and SeaSpine were granted FDA 510(k) clearance to market the Mariner Cap System, a combination of SeaSpine’s Mariner® pedicle screw system with Implanet’s JAZZ Cap® technology (shown above, left to right).
Implanet and SeaSpine co-developed Mariner Cap to facilitate the treatment of adult degenerative disorders with novel stabilization of pedicle screws. Product launch plans are underway.
Implanet and SeaSpine have been in partnership since February 2019. SeaSpine holds exclusive access and distribution rights for products co-developed with the JAZZ range under its own brand name in the U.S.
Ludovic Lastennet, CEO of Implanet, said, “The FDA clearance of the Mariner Cap System by SeaSpine is a major milestone in this partnership initiated 18 months ago, and reflects the clinical value of our proprietary JAZZ® technology. This implant, henceforth adapted to the SeaSpine Mariner® system, will be deployed over the coming months and provides a direct response to the technological innovation requirements of the world’s largest market for the treatment of spinal disorders. This new system will enable SeaSpine to provide a distinctive and high-value-added offering in this market. The success of this collaboration with the materialization of the Mariner Cap System echoes that obtained by our JAZZ Cap® solution during the first surgeries performed in the United States in complex adult deformity indications.”
Keith Valentine, President and CEO of SeaSpine, said, “The Mariner Cap System further strengthens our range of products dedicated to treating spinal disorders, and represents a proprietary alternative that we can offer our distributors, surgeons and their patients across the US. Combining our two technologies provides a unique solution for securing the stability of pedicle screw constructs. We look forward to continuing our innovative partnership with Implanet.”

You are out of free articles for this month
Subscribe as a Guest for $0 and unlock a total of 5 articles per month.
You are out of five articles for this month
Subscribe as an Executive Member for access to unlimited articles, THE ORTHOPAEDIC INDUSTRY ANNUAL REPORT and more.
JV
Julie Vetalice is ORTHOWORLD's Editorial Assistant. She has covered the orthopedic industry for over 20 years, having joined the company in 1999.







