
 Copy to clipboard
Copy to clipboard 
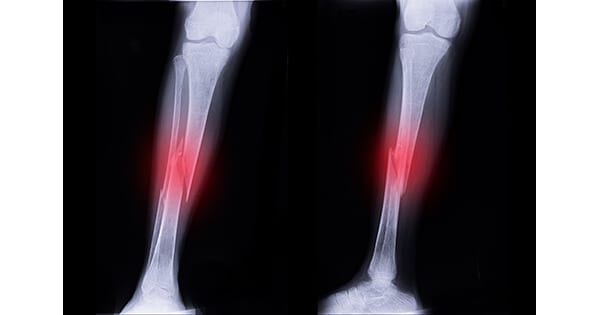
IlluminOss Medical gained expanded FDA 510(k) clearance to market its Photodynamic Bone Stabilization System for the treatment of fractures of the fibula.
This builds upon earlier indications for treatment of fractures of the pelvis, clavicle and small bones of the hands and feet, as well as traumatic, fragility, pathological and impending pathological fractures of the humerus, radius and ulna. IlluminOss can also be used in conjunction with FDA-cleared fracture fixation systems to provide supplemental fixation in all cleared anatomic sites.
The system first launched in the U.S. in 2018, having previously been commercially available ex-U.S. since 2010. Over 4,000 Photodynamic Bone Stabilization procedures have occurred to date.
“The IlluminOss technology offers a minimally invasive approach that spares soft tissues, which may lower the risk of wound complications, and can potentially drive better functional outcomes, including faster return to weight bearing and activities of daily living,” said Robert Rabiner, Chief Technology Officer of IlluminOss. “Fractures of the fibula, one of the long bones of the lower leg, are among the most common and can be particularly challenging to manage in elderly patients. Additionally, current treatment approaches such as plating have several disadvantages, including larger incisions and the potential for meaningful patient discomfort.”
IlluminOss Medical gained expanded FDA 510(k) clearance to market its Photodynamic Bone Stabilization System for the treatment of fractures of the fibula.
This builds upon earlier indications for treatment of fractures of the pelvis, clavicle and small bones of the hands and feet, as well as traumatic, fragility, pathological and impending...
IlluminOss Medical gained expanded FDA 510(k) clearance to market its Photodynamic Bone Stabilization System for the treatment of fractures of the fibula.
This builds upon earlier indications for treatment of fractures of the pelvis, clavicle and small bones of the hands and feet, as well as traumatic, fragility, pathological and impending pathological fractures of the humerus, radius and ulna. IlluminOss can also be used in conjunction with FDA-cleared fracture fixation systems to provide supplemental fixation in all cleared anatomic sites.
The system first launched in the U.S. in 2018, having previously been commercially available ex-U.S. since 2010. Over 4,000 Photodynamic Bone Stabilization procedures have occurred to date.
“The IlluminOss technology offers a minimally invasive approach that spares soft tissues, which may lower the risk of wound complications, and can potentially drive better functional outcomes, including faster return to weight bearing and activities of daily living,” said Robert Rabiner, Chief Technology Officer of IlluminOss. “Fractures of the fibula, one of the long bones of the lower leg, are among the most common and can be particularly challenging to manage in elderly patients. Additionally, current treatment approaches such as plating have several disadvantages, including larger incisions and the potential for meaningful patient discomfort.”

You are out of free articles for this month
Subscribe as a Guest for $0 and unlock a total of 5 articles per month.
You are out of five articles for this month
Subscribe as an Executive Member for access to unlimited articles, THE ORTHOPAEDIC INDUSTRY ANNUAL REPORT and more.
JV
Julie Vetalice is ORTHOWORLD's Editorial Assistant. She has covered the orthopedic industry for over 20 years, having joined the company in 1999.







