
 Copy to clipboard
Copy to clipboard 
IlluminOss Medical reached the benchmark of enrolling over 200 patients in its global device registry across 16 sites using the IlluminOss Photodynamic Bone Stabilization System.
To further the evidence of the 1000+ patients’ data in peer reviewed publications and conference proceedings, IlluminOss launched a clinical registry to demonstrate the real-world use of its device. The registry is designed to collect safety and performance data on the IlluminOss Device when used to provide stabilization and alignment for the treatment of fractures in compromised bone. The registry aims to show real world outcomes for the system. Standard of care followup visit data is collected at 75 days, six months and one year from the initial procedure. Over half of the patients enrolled have already reached their first followup visit.
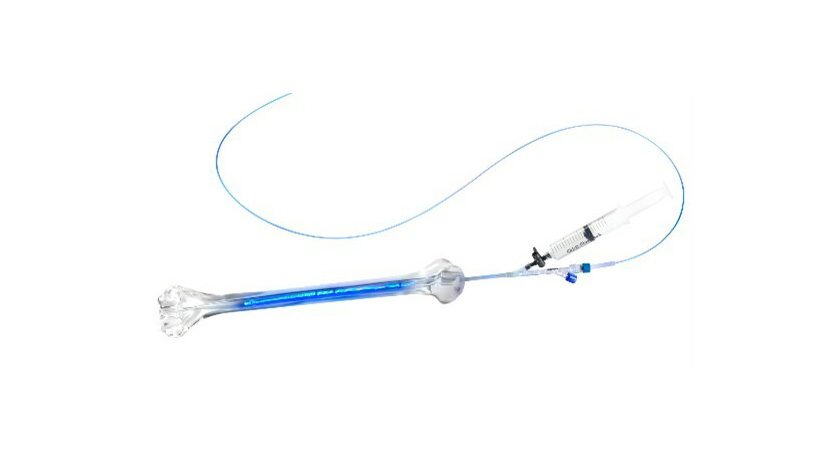
IlluminOss technology involves expanding a balloon-like implant inside a fractured bone by infusing the implant with a light-curing liquid. Blue light is then transmitted to the implant via an optical fiber to convert the liquid into a polymer, creating a strong core within the bone. This stabilizes the fracture and allows for a fast return to daily activities. Some patients are able to regain use of their injured limb the day of surgery. If additional fracture fixation is necessary, the strength supplied by the IlluminOss implant allows surgical plates and screws to be securely fastened into the bone.
Source: IlluminOss Medical, Inc.
IlluminOss Medical reached the benchmark of enrolling over 200 patients in its global device registry across 16 sites using the IlluminOss Photodynamic Bone Stabilization System.
To further the evidence of the 1000+ patients' data in peer reviewed publications and conference proceedings, IlluminOss launched a clinical registry to demonstrate...
IlluminOss Medical reached the benchmark of enrolling over 200 patients in its global device registry across 16 sites using the IlluminOss Photodynamic Bone Stabilization System.
To further the evidence of the 1000+ patients’ data in peer reviewed publications and conference proceedings, IlluminOss launched a clinical registry to demonstrate the real-world use of its device. The registry is designed to collect safety and performance data on the IlluminOss Device when used to provide stabilization and alignment for the treatment of fractures in compromised bone. The registry aims to show real world outcomes for the system. Standard of care followup visit data is collected at 75 days, six months and one year from the initial procedure. Over half of the patients enrolled have already reached their first followup visit.
IlluminOss technology involves expanding a balloon-like implant inside a fractured bone by infusing the implant with a light-curing liquid. Blue light is then transmitted to the implant via an optical fiber to convert the liquid into a polymer, creating a strong core within the bone. This stabilizes the fracture and allows for a fast return to daily activities. Some patients are able to regain use of their injured limb the day of surgery. If additional fracture fixation is necessary, the strength supplied by the IlluminOss implant allows surgical plates and screws to be securely fastened into the bone.
Source: IlluminOss Medical, Inc.

You are out of free articles for this month
Subscribe as a Guest for $0 and unlock a total of 5 articles per month.
You are out of five articles for this month
Subscribe as an Executive Member for access to unlimited articles, THE ORTHOPAEDIC INDUSTRY ANNUAL REPORT and more.
JV
Julie Vetalice is ORTHOWORLD's Editorial Assistant. She has covered the orthopedic industry for over 20 years, having joined the company in 1999.







