
 Copy to clipboard
Copy to clipboard 
FX Solutions completed enrollment for an Investigational Device Exemption study of the Easytech Reversed® Stemless implant for reverse total shoulder replacement, becoming the first company to do so.
The study, which began in December 2018, included 90 subjects across seven sites.
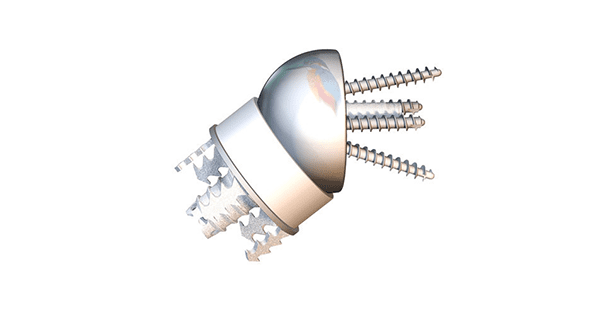
Easytech Reversed Stemless features peripheral fixation of the humeral component that is designed to be more bone-sparing vs. traditional stemmed devices.
The Easytech Reversed anchor base is designed to fit peripherally just inside of the cortical bone, with optimal bone quality for fixation. It is the first and only stemless on the market with peripheral fixation, and has been in use in the European market since 2015.
“This is not only a major milestone for FX, but for the next generation of stemless shoulder arthroplasty and the way we think about reverse total shoulder arthroplasty. As a small company, we are excited and humbled to lead the way and be the first to complete this historic study that may bring a novel device to the U.S. market which has already seen over 2000 implanted in Europe since 2015,” said Baptiste Martin, CEO of FX Shoulder USA.
Source: FX Solutions
FX Solutions completed enrollment for an Investigational Device Exemption study of the Easytech Reversed® Stemless implant for reverse total shoulder replacement, becoming the first company to do so.
The study, which began in December 2018, included 90 subjects across seven sites.
Easytech Reversed Stemless features peripheral fixation of the...
FX Solutions completed enrollment for an Investigational Device Exemption study of the Easytech Reversed® Stemless implant for reverse total shoulder replacement, becoming the first company to do so.
The study, which began in December 2018, included 90 subjects across seven sites.
Easytech Reversed Stemless features peripheral fixation of the humeral component that is designed to be more bone-sparing vs. traditional stemmed devices.
The Easytech Reversed anchor base is designed to fit peripherally just inside of the cortical bone, with optimal bone quality for fixation. It is the first and only stemless on the market with peripheral fixation, and has been in use in the European market since 2015.
“This is not only a major milestone for FX, but for the next generation of stemless shoulder arthroplasty and the way we think about reverse total shoulder arthroplasty. As a small company, we are excited and humbled to lead the way and be the first to complete this historic study that may bring a novel device to the U.S. market which has already seen over 2000 implanted in Europe since 2015,” said Baptiste Martin, CEO of FX Shoulder USA.
Source: FX Solutions

You’ve reached your limit.
We’re glad you’re finding value in our content — and we’d love for you to keep going.
Subscribe now for unlimited access to orthopedic business intelligence.
JV
Julie Vetalice is ORTHOWORLD's Editorial Assistant. She has covered the orthopedic industry for over 20 years, having joined the company in 1999.