
 Copy to clipboard
Copy to clipboard 
icotec entered into a partnership with G-21 to distribute their bone cements in conjunction with icotec’s VADER® Pedicle Screw System in the U.S.
VADER is designed to restore spinal column integrity even without fusion in patients with advanced stage tumors involving the thoracic or lumbar spine in whom life expectance, prior to oncological treatment, is of insufficient duration to permit achievement of fusion. VADER gained FDA 510(k) clearance in November 2020.
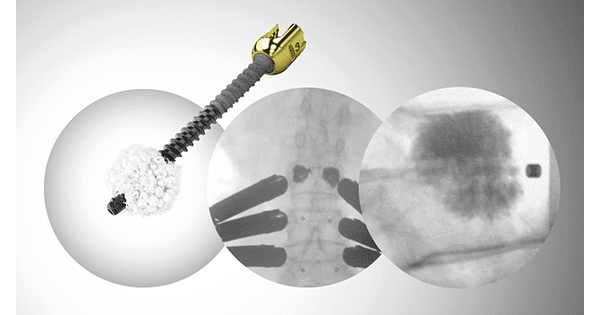
Screw fenestration allows for bone cement augmentation following icotec’s open, minimally invasive, or percutaneous surgical procedures to increase stability.
G-21 manufactures V-Steady and V-Fast polymethylmethacrylate-based bone cements formulated for delivery via vertebral augmentation. When used with the icotec VADER pedicle screw 6.0, V-Steady and V-Fast cements can increase stability in patients with compromised bone quality as long as the integrity of the spine is not severely compromised.
“The G-21 bone cements are a perfect complement to icotec’s fast growing portfolio of spinal implants made from our proprietary radiolucent, nonmetallic BlackArmor® material that produces minimal image artifact while eliminating shielding and scattering of radiation during oncology treatments,” said Roger Stadler, CEO of icotec ag.
Filippo Foroni, Executive Vice President of G-21, said, “This distribution agreement is a significant milestone for the growth of G-21 and the sale of our bone cements for pedicle screw augmentation in the United States. icotec is an essential partner that has the corporate expertise, sales network and marketing reach to bring our combined solution to spine surgeons and their spinal tumor patients across the country.”
icotec entered into a partnership with G-21 to distribute their bone cements in conjunction with icotec's VADER® Pedicle Screw System in the U.S.
VADER is designed to restore spinal column integrity even without fusion in patients with advanced stage tumors involving the thoracic or lumbar spine in whom life expectance, prior to oncological...
icotec entered into a partnership with G-21 to distribute their bone cements in conjunction with icotec’s VADER® Pedicle Screw System in the U.S.
VADER is designed to restore spinal column integrity even without fusion in patients with advanced stage tumors involving the thoracic or lumbar spine in whom life expectance, prior to oncological treatment, is of insufficient duration to permit achievement of fusion. VADER gained FDA 510(k) clearance in November 2020.
Screw fenestration allows for bone cement augmentation following icotec’s open, minimally invasive, or percutaneous surgical procedures to increase stability.
G-21 manufactures V-Steady and V-Fast polymethylmethacrylate-based bone cements formulated for delivery via vertebral augmentation. When used with the icotec VADER pedicle screw 6.0, V-Steady and V-Fast cements can increase stability in patients with compromised bone quality as long as the integrity of the spine is not severely compromised.
“The G-21 bone cements are a perfect complement to icotec’s fast growing portfolio of spinal implants made from our proprietary radiolucent, nonmetallic BlackArmor® material that produces minimal image artifact while eliminating shielding and scattering of radiation during oncology treatments,” said Roger Stadler, CEO of icotec ag.
Filippo Foroni, Executive Vice President of G-21, said, “This distribution agreement is a significant milestone for the growth of G-21 and the sale of our bone cements for pedicle screw augmentation in the United States. icotec is an essential partner that has the corporate expertise, sales network and marketing reach to bring our combined solution to spine surgeons and their spinal tumor patients across the country.”

You are out of free articles for this month
Subscribe as a Guest for $0 and unlock a total of 5 articles per month.
You are out of five articles for this month
Subscribe as an Executive Member for access to unlimited articles, THE ORTHOPAEDIC INDUSTRY ANNUAL REPORT and more.
JV
Julie Vetalice is ORTHOWORLD's Editorial Assistant. She has covered the orthopedic industry for over 20 years, having joined the company in 1999.







