
 Copy to clipboard
Copy to clipboard 
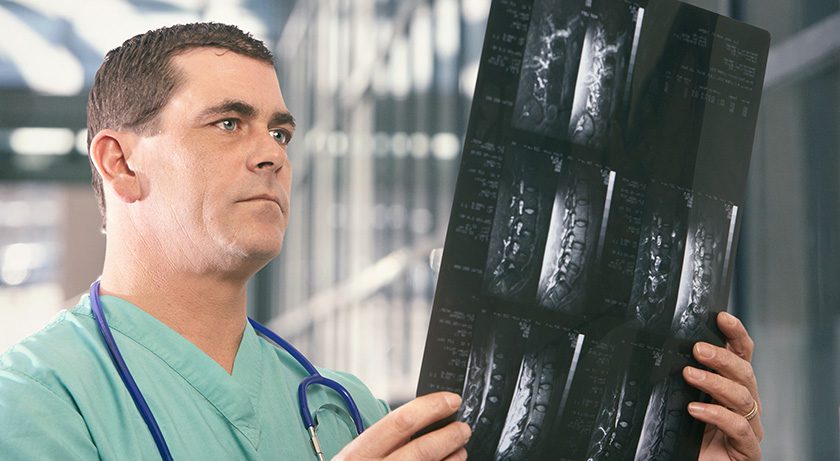
icotec received FDA clearance for the use of BlackArmor implants in the treatment of de novo spinal infections.
icotec is reported to be the first and only company in the U.S. with FDA 510(k) clearance for stabilizing the spine in de novo spinal infections, including discitis, osteomyelitis, pyogenic infection of the intervertebral disc and other spondylopathies.
In addition to the on-label designation, FDA has also granted a Breakthrough Device Designation for this indication across the entirety of icotec’s BlackArmor spinal stabilization portfolio.
Furthermore, following the FDA decision, Centers for Medicare and Medicaid Services approved BlackArmor for New Technology Add-on Payment (NTAP), which is awarded when medical technologies are determined to significantly improve the diagnosis or treatment of Medicare beneficiaries. As of October 1, 2024, hospitals are eligible to receive additional payments for the VADER pedicle screw system of up to $28,000 for Medicare Fee-for-Service patients.
icotec’s BlackArmor implants offer reduced artifacts from radiolucent Carbon/PEEK material, which enables improved imaging in the post-operative setting as well as in the monitoring of infection.
“Over 15,000 patients receive spinal stabilizations due to an infection in the spine in the USA every year,” says Chris Eigenmann, CEO icotec Medical US. “Being able to help these patients with an implant that allows for improved post-operative monitoring and visualization is a great opportunity and privilege.”
Source: icotec
icotec received FDA clearance for the use of BlackArmor implants in the treatment of de novo spinal infections.
icotec is reported to be the first and only company in the U.S. with FDA 510(k) clearance for stabilizing the spine in de novo spinal infections, including discitis, osteomyelitis, pyogenic infection of the intervertebral disc and...
icotec received FDA clearance for the use of BlackArmor implants in the treatment of de novo spinal infections.
icotec is reported to be the first and only company in the U.S. with FDA 510(k) clearance for stabilizing the spine in de novo spinal infections, including discitis, osteomyelitis, pyogenic infection of the intervertebral disc and other spondylopathies.
In addition to the on-label designation, FDA has also granted a Breakthrough Device Designation for this indication across the entirety of icotec’s BlackArmor spinal stabilization portfolio.
Furthermore, following the FDA decision, Centers for Medicare and Medicaid Services approved BlackArmor for New Technology Add-on Payment (NTAP), which is awarded when medical technologies are determined to significantly improve the diagnosis or treatment of Medicare beneficiaries. As of October 1, 2024, hospitals are eligible to receive additional payments for the VADER pedicle screw system of up to $28,000 for Medicare Fee-for-Service patients.
icotec’s BlackArmor implants offer reduced artifacts from radiolucent Carbon/PEEK material, which enables improved imaging in the post-operative setting as well as in the monitoring of infection.
“Over 15,000 patients receive spinal stabilizations due to an infection in the spine in the USA every year,” says Chris Eigenmann, CEO icotec Medical US. “Being able to help these patients with an implant that allows for improved post-operative monitoring and visualization is a great opportunity and privilege.”
Source: icotec

You are out of free articles for this month
Subscribe as a Guest for $0 and unlock a total of 5 articles per month.
You are out of five articles for this month
Subscribe as an Executive Member for access to unlimited articles, THE ORTHOPAEDIC INDUSTRY ANNUAL REPORT and more.
JV
Julie Vetalice is ORTHOWORLD's Editorial Assistant. She has covered the orthopedic industry for over 20 years, having joined the company in 1999.







