
 Copy to clipboard
Copy to clipboard 
icotec received FDA 510(k) clearance to market the CMORE CT System for use in the cervicothoracic spine. U.S. launch is impending.
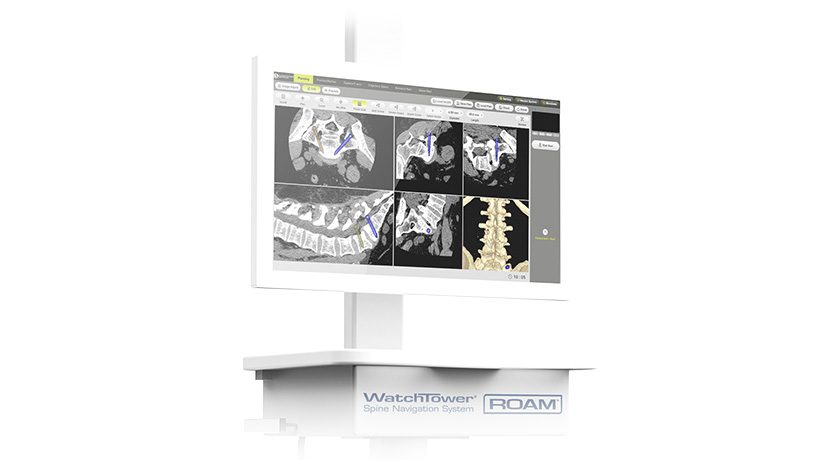
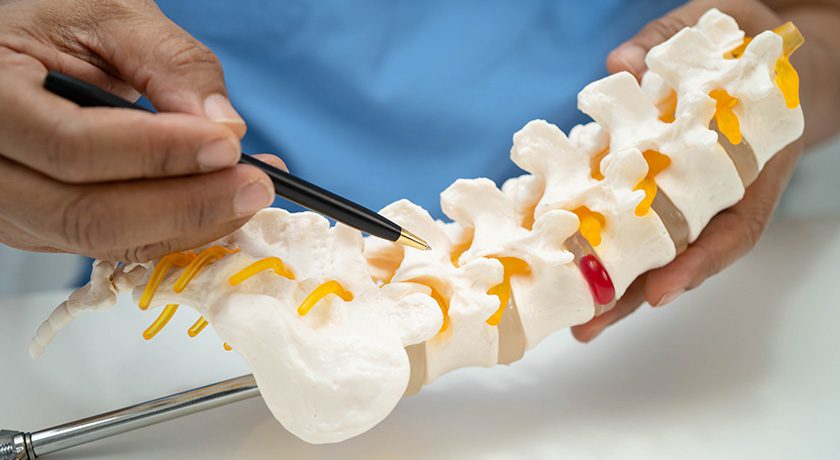
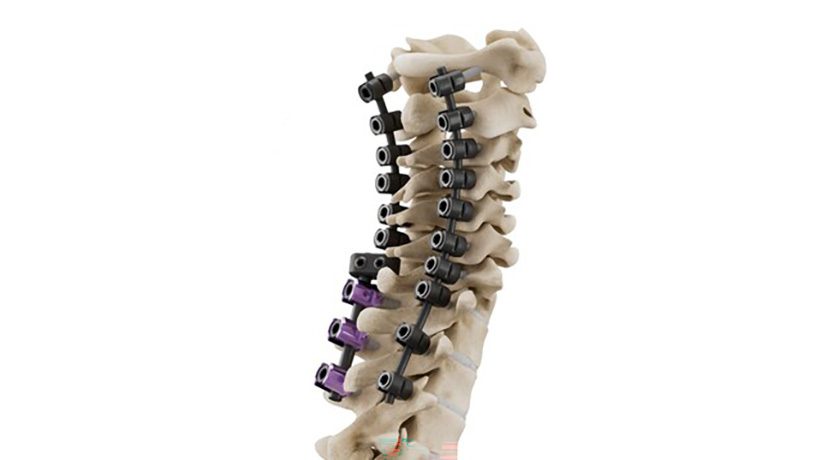
CMORE is an enhanced set of instruments and implants for posterior stabilization of the upper spine. The spinal implants are made of non-metallic and radiolucent BlackArmor technology, enabling a spectrum of treatment modalities in adjuvant tumor therapy and enhancing postoperative imaging diagnostics in the cervicothoracic spine. Comprising a full portfolio of polyaxial screws, straight and precontoured rods, axial and parallel connectors, the CMORE CT System provides the versatility needed to accommodate variations in patients’ anatomy and pathology.
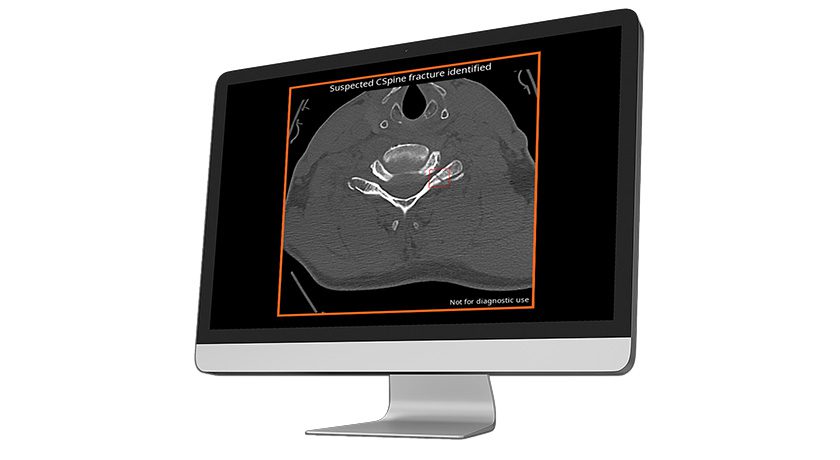
BlackArmor Engineered Carbon/PEEK is a combination composite material with continuous, high strength carbon-fibers and PEEK, manufactured in a process developed by icotec. The result is an implant component with an interwoven 3D fiber architecture. It is also radiolucent in all diagnostic imaging modes (MRI, CT, and X-ray).
“The FDA clearance of the CMORE® CT System marks another milestone in our commitment to advancing care for patients with spinal tumors, infections, and complex pathologies,” said Christoph Eigenmann, CEO of icotec Medical, U.S. “By expanding our BlackArmor® technology to the posterior cervical spine, we’re giving surgeons and radiation oncologists a new level of visibility and confidence in their postoperative and oncological decision-making.”
Source: icotec
icotec received FDA 510(k) clearance to market the CMORE CT System for use in the cervicothoracic spine. U.S. launch is impending.
CMORE is an enhanced set of instruments and implants for posterior stabilization of the upper spine. The spinal implants are made of non-metallic and radiolucent BlackArmor technology, enabling a spectrum of...
icotec received FDA 510(k) clearance to market the CMORE CT System for use in the cervicothoracic spine. U.S. launch is impending.
CMORE is an enhanced set of instruments and implants for posterior stabilization of the upper spine. The spinal implants are made of non-metallic and radiolucent BlackArmor technology, enabling a spectrum of treatment modalities in adjuvant tumor therapy and enhancing postoperative imaging diagnostics in the cervicothoracic spine. Comprising a full portfolio of polyaxial screws, straight and precontoured rods, axial and parallel connectors, the CMORE CT System provides the versatility needed to accommodate variations in patients’ anatomy and pathology.
BlackArmor Engineered Carbon/PEEK is a combination composite material with continuous, high strength carbon-fibers and PEEK, manufactured in a process developed by icotec. The result is an implant component with an interwoven 3D fiber architecture. It is also radiolucent in all diagnostic imaging modes (MRI, CT, and X-ray).
“The FDA clearance of the CMORE® CT System marks another milestone in our commitment to advancing care for patients with spinal tumors, infections, and complex pathologies,” said Christoph Eigenmann, CEO of icotec Medical, U.S. “By expanding our BlackArmor® technology to the posterior cervical spine, we’re giving surgeons and radiation oncologists a new level of visibility and confidence in their postoperative and oncological decision-making.”
Source: icotec

You’ve reached your limit.
We’re glad you’re finding value in our content — and we’d love for you to keep going.
Subscribe now for unlimited access to orthopedic business intelligence.
JV
Julie Vetalice is ORTHOWORLD's Editorial Assistant. She has covered the orthopedic industry for over 20 years, having joined the company in 1999.