
 Copy to clipboard
Copy to clipboard 
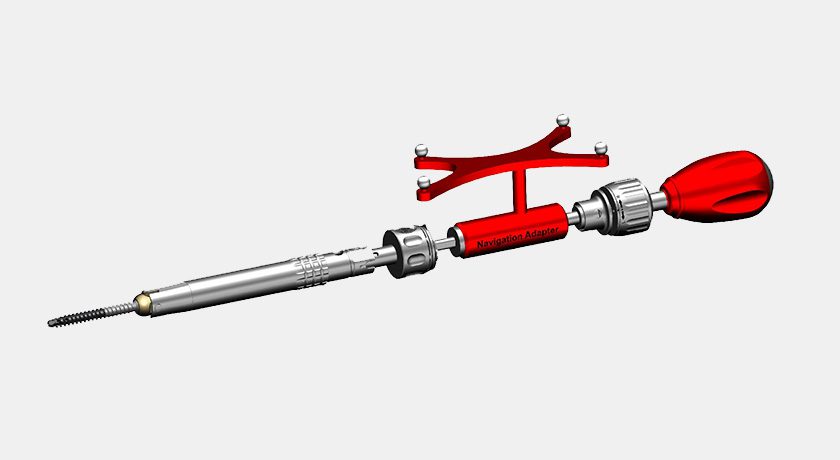
icotec received FDA 510(k) clearance to market VADER Pedicle System Navigated Instruments. The newly-cleared Navigation set includes a comprehensive range of instruments for both 0pen and MIS surgery.
“Receiving FDA 510(k) clearance for our navigated instrumentation is a significant achievement for icotec,” said Roger Stadler, CEO at icotec. “We are proud to offer our surgeons the tools they need to perform these procedures with the utmost precision and confidence. This clearance opens new opportunities for growth and reaffirms our position as the leader in Carbon/PEEK implants and our commitment to keeping a standard surgical technique.”
Source: icotec
icotec received FDA 510(k) clearance to market VADER Pedicle System Navigated Instruments. The newly-cleared Navigation set includes a comprehensive range of instruments for both 0pen and MIS surgery.
“Receiving FDA 510(k) clearance for our navigated instrumentation is a significant achievement for icotec,” said Roger Stadler, CEO at icotec....
icotec received FDA 510(k) clearance to market VADER Pedicle System Navigated Instruments. The newly-cleared Navigation set includes a comprehensive range of instruments for both 0pen and MIS surgery.
“Receiving FDA 510(k) clearance for our navigated instrumentation is a significant achievement for icotec,” said Roger Stadler, CEO at icotec. “We are proud to offer our surgeons the tools they need to perform these procedures with the utmost precision and confidence. This clearance opens new opportunities for growth and reaffirms our position as the leader in Carbon/PEEK implants and our commitment to keeping a standard surgical technique.”
Source: icotec

You are out of free articles for this month
Subscribe as a Guest for $0 and unlock a total of 5 articles per month.
You are out of five articles for this month
Subscribe as an Executive Member for access to unlimited articles, THE ORTHOPAEDIC INDUSTRY ANNUAL REPORT and more.
JV
Julie Vetalice is ORTHOWORLD's Editorial Assistant. She has covered the orthopedic industry for over 20 years, having joined the company in 1999.







