Copy to clipboard
Copy to clipboard 
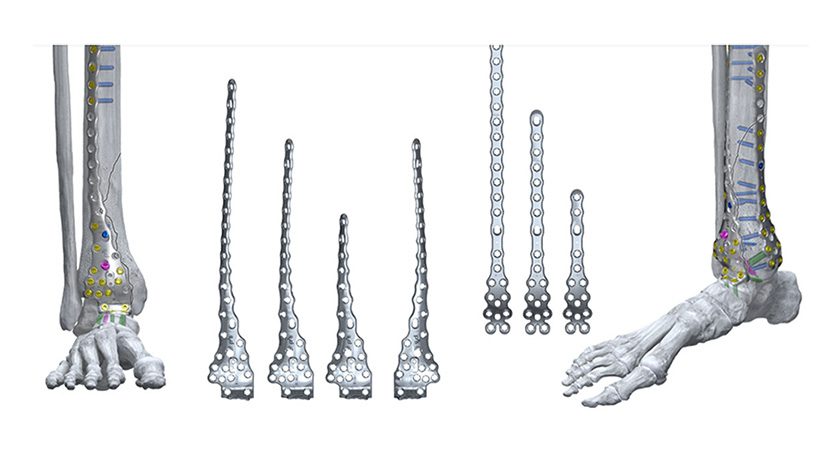
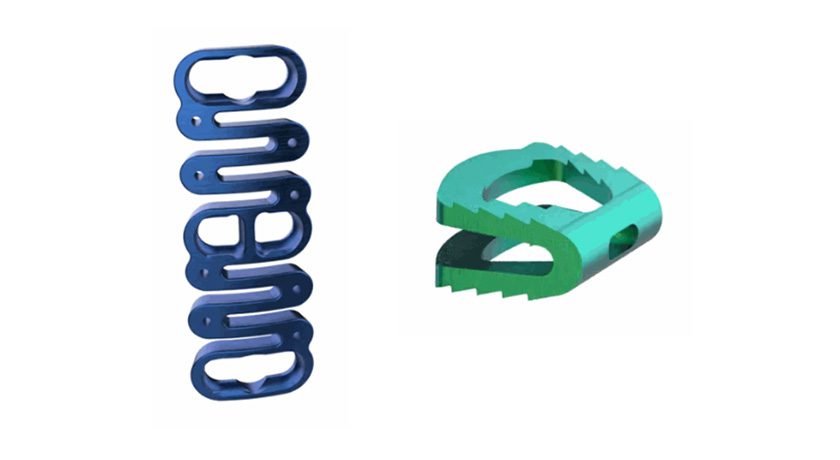
Hyprevention has developed the STRUTPLASTY Technology to reinforce bones weakened by osteoporosis or cancer. The Y-STRUT Hip Implant, part of STRUTPLASTY, has been recognized as a Breakthrough Device by FDA.
Y-STRUT is cleared for the following indications:
- Oncology: For adult patients with cancer presenting with bone metastasis or lesions in the proximal femur. It is intended for percutaneous prophylactic fixation of the proximal femur in patients with impending pathological fractures.
- Orthopedics/Traumatology: For contralateral percutaneous internal fixation of the proximal femur in osteoporotic patients with a low-energy per trochanteric fracture on one side and at risk of a new hip fracture.
Cecile Vienney Vivez, CEO and founder of Hyprevention, has 25 years of experience in developing innovative orthopedic products at Stryker and in start-ups like Vexim (now part of Stryker) and Hyprevention. She stated, “Hip fracture prevention is crucial for the elderly and patients with tumors in the proximal femur to ensure the best quality of life. Y-STRUT was the first product developed at Hyprevention, as the hip is one of the most common sites of bone fractures, with the spine. After marketing the product in Europe, we are excited to introduce the Y-STRUT Technology in the United States. Clinical studies conducted in France and Belgium have demonstrated the safety and efficacy of our product, making numerous patients happy and autonomous. Receiving FDA Breakthrough Designation for our hip device is a major recognition for the company and the team.”
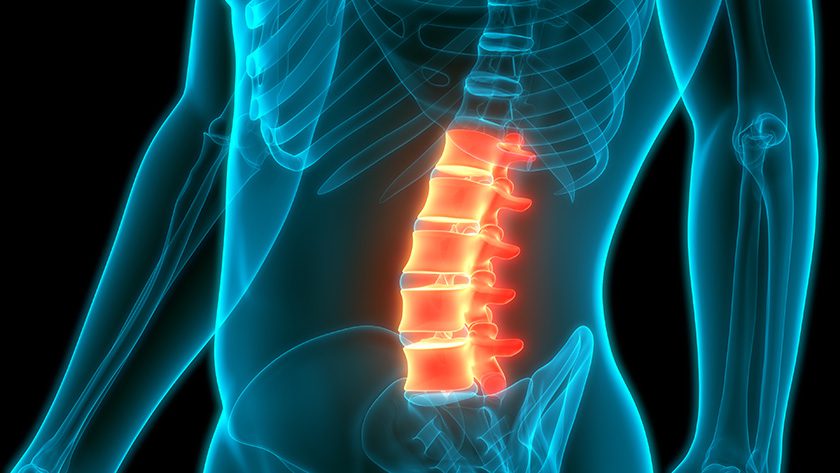
Hyprevention is building a product portfolio with its STRUTPLASTY Technology. The V-STRUT Vertebral Implant, indicated for treating vertebral fractures, is already available in the U.S. market following two FDA 510(k) clearances. With the new Y-STRUT Hip Implant, the company confirms its ability to meet regulatory requirements and address the clinical needs of patients and physicians.
Source: Hyprevention
Hyprevention has developed the STRUTPLASTY Technology to reinforce bones weakened by osteoporosis or cancer. The Y-STRUT Hip Implant, part of STRUTPLASTY, has been recognized as a Breakthrough Device by FDA.
Y-STRUT is cleared for the following indications:
Oncology: For adult patients with cancer presenting with bone metastasis or...
Hyprevention has developed the STRUTPLASTY Technology to reinforce bones weakened by osteoporosis or cancer. The Y-STRUT Hip Implant, part of STRUTPLASTY, has been recognized as a Breakthrough Device by FDA.
Y-STRUT is cleared for the following indications:
- Oncology: For adult patients with cancer presenting with bone metastasis or lesions in the proximal femur. It is intended for percutaneous prophylactic fixation of the proximal femur in patients with impending pathological fractures.
- Orthopedics/Traumatology: For contralateral percutaneous internal fixation of the proximal femur in osteoporotic patients with a low-energy per trochanteric fracture on one side and at risk of a new hip fracture.
Cecile Vienney Vivez, CEO and founder of Hyprevention, has 25 years of experience in developing innovative orthopedic products at Stryker and in start-ups like Vexim (now part of Stryker) and Hyprevention. She stated, “Hip fracture prevention is crucial for the elderly and patients with tumors in the proximal femur to ensure the best quality of life. Y-STRUT was the first product developed at Hyprevention, as the hip is one of the most common sites of bone fractures, with the spine. After marketing the product in Europe, we are excited to introduce the Y-STRUT Technology in the United States. Clinical studies conducted in France and Belgium have demonstrated the safety and efficacy of our product, making numerous patients happy and autonomous. Receiving FDA Breakthrough Designation for our hip device is a major recognition for the company and the team.”
Hyprevention is building a product portfolio with its STRUTPLASTY Technology. The V-STRUT Vertebral Implant, indicated for treating vertebral fractures, is already available in the U.S. market following two FDA 510(k) clearances. With the new Y-STRUT Hip Implant, the company confirms its ability to meet regulatory requirements and address the clinical needs of patients and physicians.
Source: Hyprevention

You are out of free articles for this month
Subscribe as a Guest for $0 and unlock a total of 5 articles per month.
You are out of five articles for this month
Subscribe as an Executive Member for access to unlimited articles, THE ORTHOPAEDIC INDUSTRY ANNUAL REPORT and more.
JV
Julie Vetalice is ORTHOWORLD's Editorial Assistant. She has covered the orthopedic industry for over 20 years, having joined the company in 1999.