
 Copy to clipboard
Copy to clipboard 
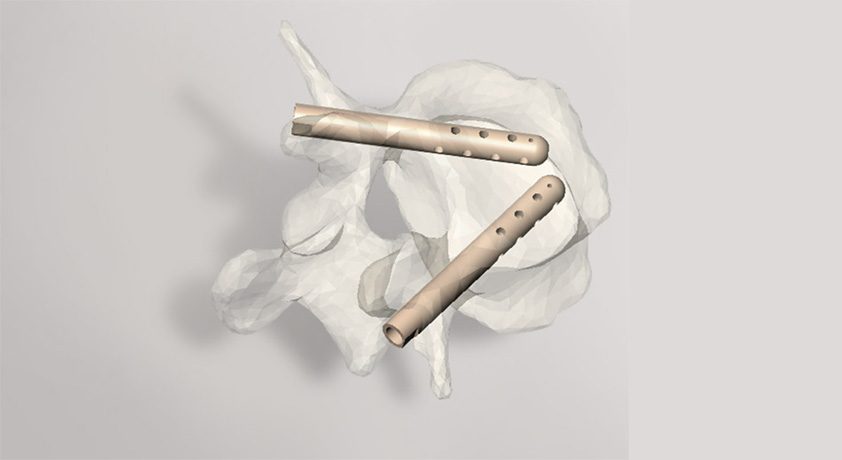
Hyprevention has incorporated in Delaware, becoming the new parent company of the group previously established in France in 2010. Hyprevention develops and markets the Strutplasty Technology, an FDA 510(k)-cleared device under the trade name V-Strut Transpedicular Vertebral Implant. The technology, which combines a PEEK implant with bone cement, reinforces weakened bone due to osteoporosis or cancer in the vertebral body. Initial U.S. commercialization has demonstrated strong clinical outcomes and growth.
Cecile Vienney, CEO and founder of Hyprevention, said, “Establishing Hyprevention as a U.S. company will allow us to be laser focused on growing the U.S. sales team and bringing a next generation product to physicians that treat patients with vertebral fractures. I founded Hyprevention to solve problems in the current standard of care addressing vertebral fractures.”
In addition to establishing Hyprevention as a U.S.-based product, the company appointed Kathryn Larson to the Board of Directors. “Kathryn brings a wealth of commercial knowledge and experience to Hyprevention, including her most recent success with Vertiflex (acquired by Boston Scientific Inc). Kathryn will help further the U.S. launch of V-Strut and provide direction to our commercial teams.”
Source: Hyprevention Inc.
Hyprevention has incorporated in Delaware, becoming the new parent company of the group previously established in France in 2010. Hyprevention develops and markets the Strutplasty Technology, an FDA 510(k)-cleared device under the trade name V-Strut Transpedicular Vertebral Implant. The technology, which combines a PEEK implant with bone...
Hyprevention has incorporated in Delaware, becoming the new parent company of the group previously established in France in 2010. Hyprevention develops and markets the Strutplasty Technology, an FDA 510(k)-cleared device under the trade name V-Strut Transpedicular Vertebral Implant. The technology, which combines a PEEK implant with bone cement, reinforces weakened bone due to osteoporosis or cancer in the vertebral body. Initial U.S. commercialization has demonstrated strong clinical outcomes and growth.
Cecile Vienney, CEO and founder of Hyprevention, said, “Establishing Hyprevention as a U.S. company will allow us to be laser focused on growing the U.S. sales team and bringing a next generation product to physicians that treat patients with vertebral fractures. I founded Hyprevention to solve problems in the current standard of care addressing vertebral fractures.”
In addition to establishing Hyprevention as a U.S.-based product, the company appointed Kathryn Larson to the Board of Directors. “Kathryn brings a wealth of commercial knowledge and experience to Hyprevention, including her most recent success with Vertiflex (acquired by Boston Scientific Inc). Kathryn will help further the U.S. launch of V-Strut and provide direction to our commercial teams.”
Source: Hyprevention Inc.

You are out of free articles for this month
Subscribe as a Guest for $0 and unlock a total of 5 articles per month.
You are out of five articles for this month
Subscribe as an Executive Member for access to unlimited articles, THE ORTHOPAEDIC INDUSTRY ANNUAL REPORT and more.
JV
Julie Vetalice is ORTHOWORLD's Editorial Assistant. She has covered the orthopedic industry for over 20 years, having joined the company in 1999.







