
 Copy to clipboard
Copy to clipboard 
Hy2Care completed the second phase of its clinical trial with the treatment of the 46th patient using the CartRevive hydrogel implant. In early 2023, the Medical Ethical Review Committee of UMC Utrecht granted approval to continue the clinical study with an additional group of 36 patients, following a successful first safety group of 10 patients that started in 2022.
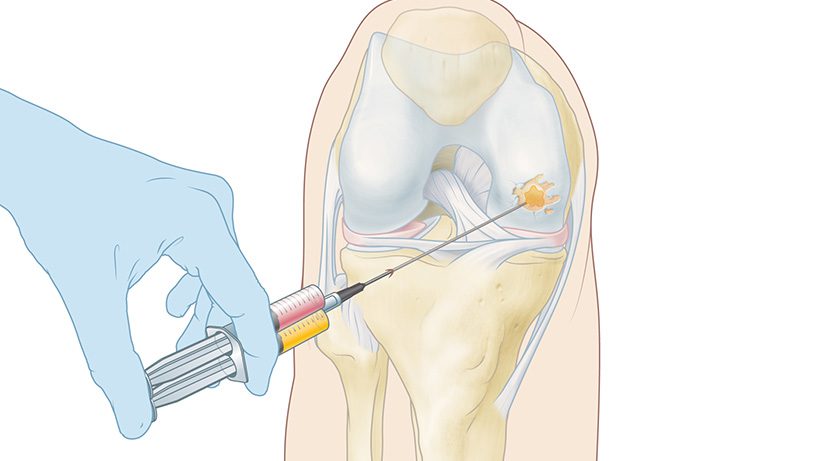
The CartRevive hydrogel implant is designed to enable optimal cartilage repair in the knee, addressing an unmet clinical need due to the high prevalence of cartilage repair surgeries worldwide.
The next step is expanding into the US market, where Hy2Care is preparing to submit an Investigational Device Exemption by the end of 2024. The IDE will pave the way for the US clinical trial, with the first patient treatment expected in early 2026. This milestone aligns with Hy2Care’s European goals, as CE marking is anticipated also by early 2026. Upon receiving CE approval, Hy2Care will begin commercialization and initiate reimbursement trials in various European countries.
Leo Smit, CEO of Hy2Care, said, “Treating our 46th patient is a major achievement in our mission to bring the Hy2Care CartRevive hydrogel implant to patients worldwide. The first real experiences with applying this new therapy strengthen our conviction that we are on the right track of delivering a new solution that improves patient outcomes and will have a positive influence in the daily lives of many people.”
Source: Hy2Care
Hy2Care completed the second phase of its clinical trial with the treatment of the 46th patient using the CartRevive hydrogel implant. In early 2023, the Medical Ethical Review Committee of UMC Utrecht granted approval to continue the clinical study with an additional group of 36 patients, following a successful first safety group of 10 patients...
Hy2Care completed the second phase of its clinical trial with the treatment of the 46th patient using the CartRevive hydrogel implant. In early 2023, the Medical Ethical Review Committee of UMC Utrecht granted approval to continue the clinical study with an additional group of 36 patients, following a successful first safety group of 10 patients that started in 2022.
The CartRevive hydrogel implant is designed to enable optimal cartilage repair in the knee, addressing an unmet clinical need due to the high prevalence of cartilage repair surgeries worldwide.
The next step is expanding into the US market, where Hy2Care is preparing to submit an Investigational Device Exemption by the end of 2024. The IDE will pave the way for the US clinical trial, with the first patient treatment expected in early 2026. This milestone aligns with Hy2Care’s European goals, as CE marking is anticipated also by early 2026. Upon receiving CE approval, Hy2Care will begin commercialization and initiate reimbursement trials in various European countries.
Leo Smit, CEO of Hy2Care, said, “Treating our 46th patient is a major achievement in our mission to bring the Hy2Care CartRevive hydrogel implant to patients worldwide. The first real experiences with applying this new therapy strengthen our conviction that we are on the right track of delivering a new solution that improves patient outcomes and will have a positive influence in the daily lives of many people.”
Source: Hy2Care

You are out of free articles for this month
Subscribe as a Guest for $0 and unlock a total of 5 articles per month.
You are out of five articles for this month
Subscribe as an Executive Member for access to unlimited articles, THE ORTHOPAEDIC INDUSTRY ANNUAL REPORT and more.
JV
Julie Vetalice is ORTHOWORLD's Editorial Assistant. She has covered the orthopedic industry for over 20 years, having joined the company in 1999.







