
 Copy to clipboard
Copy to clipboard 
Histogenics completed patient enrollment of its NeoCart® Phase III clinical trial, which is being conducted under a Special Protocol Assessment with FDA.
The 245-patient trial is evaluating pain and function improvement of patients treated with NeoCart tissue engineered implant compared to microfracture. Histogenics expects to report top-line 1-year superiority data from the trial in 3Q18, followed by a Biologics License Application, potential approval and 2H19 market launch.
Results from Phase I and Phase II study indicated that NeoCart is a safe, effective treatment for articular cartilage lesions through 5-year follow-up. The Phase III trial update follows the announcement in May that Histogenics successfully completed consultations with Japan Pharmaceuticals and Medical Devices Agency regarding the regulatory path for NeoCart.
Sources: Histogenics; ORTHOWORLD Inc.
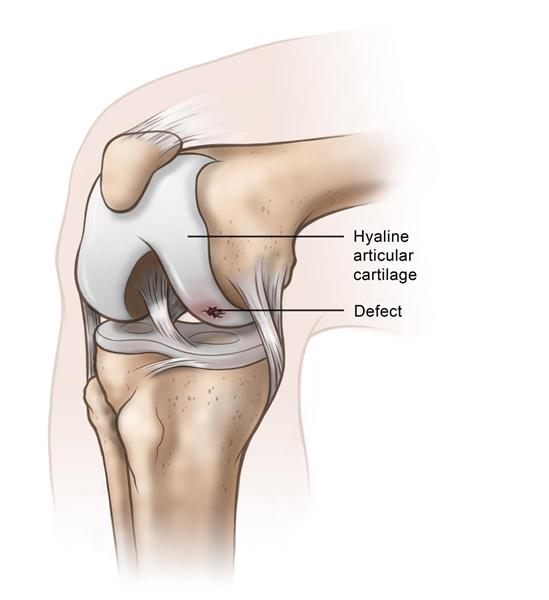
Photo courtesy of Histogenics
Histogenics completed patient enrollment of its NeoCart® Phase III clinical trial, which is being conducted under a Special Protocol Assessment with FDA.
The 245-patient trial is evaluating pain and function improvement of patients treated with NeoCart tissue engineered implant compared to microfracture. Histogenics expects to report top-line...
Histogenics completed patient enrollment of its NeoCart® Phase III clinical trial, which is being conducted under a Special Protocol Assessment with FDA.
The 245-patient trial is evaluating pain and function improvement of patients treated with NeoCart tissue engineered implant compared to microfracture. Histogenics expects to report top-line 1-year superiority data from the trial in 3Q18, followed by a Biologics License Application, potential approval and 2H19 market launch.
Results from Phase I and Phase II study indicated that NeoCart is a safe, effective treatment for articular cartilage lesions through 5-year follow-up. The Phase III trial update follows the announcement in May that Histogenics successfully completed consultations with Japan Pharmaceuticals and Medical Devices Agency regarding the regulatory path for NeoCart.
Sources: Histogenics; ORTHOWORLD Inc.

Photo courtesy of Histogenics

You are out of free articles for this month
Subscribe as a Guest for $0 and unlock a total of 5 articles per month.
You are out of five articles for this month
Subscribe as an Executive Member for access to unlimited articles, THE ORTHOPAEDIC INDUSTRY ANNUAL REPORT and more.
JV
Julie Vetalice is ORTHOWORLD's Editorial Assistant. She has covered the orthopedic industry for over 20 years, having joined the company in 1999.







