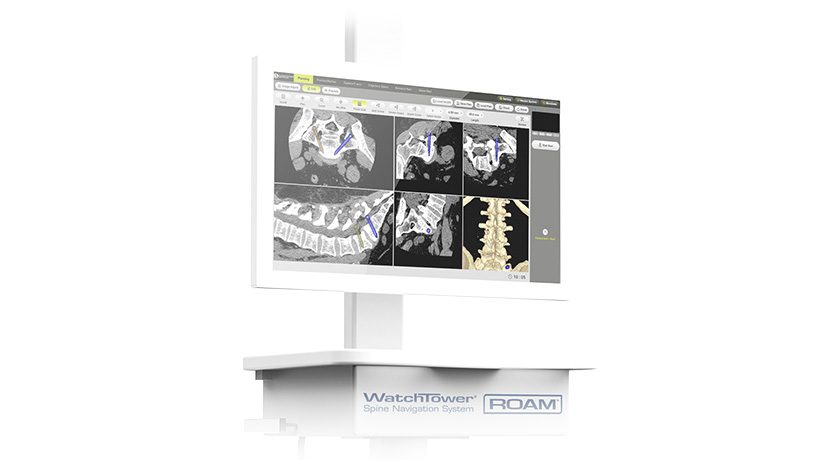
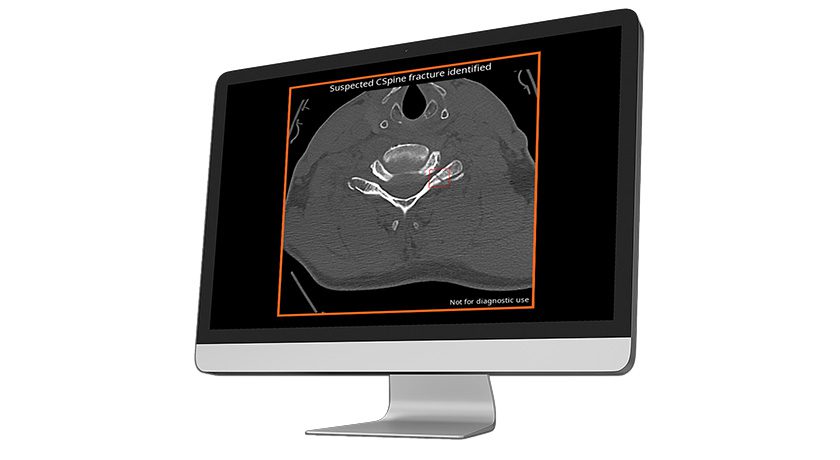
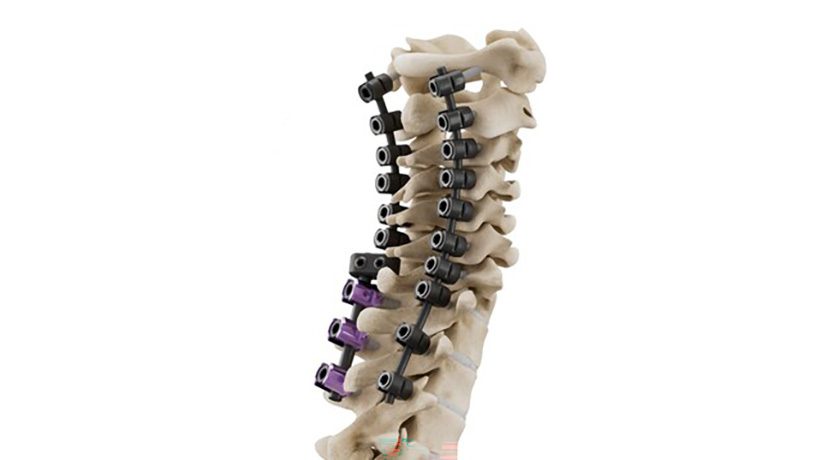
Copy to clipboard
Copy to clipboard 
Histogenics has enrolled 123 (>50%) of the 245 patients required to complete enrollment in its ongoing NeoCart® Phase III clinical trial. This trial is being conducted under a Special Protocol Assessment with FDA.
Company leadership attributes its progress to enrollment-enhancing strategies implemented in 2015, as well as changes by FDA in 4Q15 to the Phase III clinical trial inclusion/exclusion criteria. Trial enrollment is expected to complete in 2Q17.
The trial is investigating NeoCart autologous cell therapy vs. microfracture as a first-line approach for the treatment of full thickness adult knee cartilage defects.
Source: Histogenics Corporation
Histogenics has enrolled 123 (>50%) of the 245 patients required to complete enrollment in its ongoing NeoCart® Phase III clinical trial. This trial is being conducted under a Special Protocol Assessment with FDA.
Company leadership attributes its progress to enrollment-enhancing strategies implemented in 2015, as well as changes by FDA in...
Histogenics has enrolled 123 (>50%) of the 245 patients required to complete enrollment in its ongoing NeoCart® Phase III clinical trial. This trial is being conducted under a Special Protocol Assessment with FDA.
Company leadership attributes its progress to enrollment-enhancing strategies implemented in 2015, as well as changes by FDA in 4Q15 to the Phase III clinical trial inclusion/exclusion criteria. Trial enrollment is expected to complete in 2Q17.
The trial is investigating NeoCart autologous cell therapy vs. microfracture as a first-line approach for the treatment of full thickness adult knee cartilage defects.
Source: Histogenics Corporation

You’ve reached your limit.
We’re glad you’re finding value in our content — and we’d love for you to keep going.
Subscribe now for unlimited access to orthopedic business intelligence.
JV
Julie Vetalice is ORTHOWORLD's Editorial Assistant. She has covered the orthopedic industry for over 20 years, having joined the company in 1999.