Copy to clipboard
Copy to clipboard 
Hip replacement has evolved significantly since the earliest attempts, which occurred in the late 19th century and employed materials like ivory and glass. How far we’ve come.
For 2024, hip replacement revenue reached $8.75 billion with growth of 4.5% vs. 2023. ORTHOWORLD estimates that growth arose from ongoing procedure recovery from prior years, as well as developing tailwinds. Here are four implant and technology developments that emerged in the past quarter to further advance modern total hip replacement.
First Clinical Use of Corin’s ApolloHipX
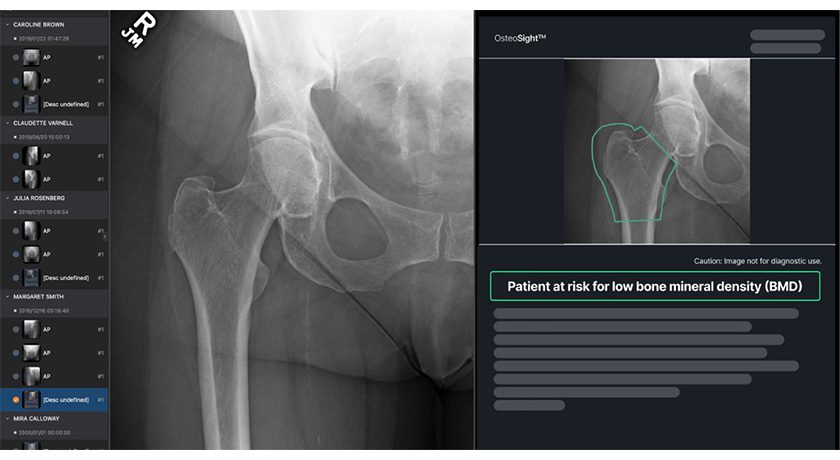
 ApolloHipX is the first total hip arthroplasty solution to combine dynamic 3D preoperative planning with real-time intraoperative assessment from standard fluoroscopy.
ApolloHipX is the first total hip arthroplasty solution to combine dynamic 3D preoperative planning with real-time intraoperative assessment from standard fluoroscopy.
ApolloHipX integrates Corin’s preoperative planning solution with intraoperative fluoroscopy via proprietary, automated 2D–3D registration software. By aligning a single 2D x-ray with the patient-specific 3D plan, the system provides a real-time 3D assessment of implant position, regardless of patient positioning or C-arm alignment. This streamlined approach to fluoroscopy-based THA reduces the number of intraoperative images required, minimizing surgical time and radiation exposure within the O.R.
Apollo was built as a connected surgical ecosystem — integrating AI-driven planning, intraoperative tools and cloud-based analytics to support more consistent, personalized orthopedic care. ApolloKnee, introduced in 2024, brought pre-resection balance assessment into the operating room with BalanceBot, enabling data-driven implant decisions before bone cuts are made. ApolloHipX now extends that same foundation to total hip replacement, offering real-time 3D validation within a streamlined, fluoroscopy-based workflow.
First Patient Treated with Alteon Short Tapered Wedge Hip

Exactech announced the first surgery using the new Alteon Short Tapered Wedge Hip Stem. Currently in limited launch across select U.S. sites, full U.S. release is slated for 4Q25.
Engineered to conserve distal bone, the new stem incorporates features designed to achieve immediate axial and rotational stability while reproducibly creating the desired leg length and offset.
Compared to previous generation wedge stems, the Alteon Tapered Wedge Stems intentionally grow at a smaller rate laterally and distally, especially in the smaller stem sizes. In addition, the Short Tapered Wedge is up to 25% shorter than standard Tapered Wedge stems.
JointMedica Launches Investigational Device Exemption Study for Polymotion Hip Resurfacing
The study aims to evaluate the system’s safety and clinical effectiveness in addressing the challenges faced by younger, more active patients.
Polymotion Hip Resurfacing conserves bone by removing less of the femoral head than traditional hip replacement, reshaping it to accommodate a metal cap that mirrors the natural anatomy. A cup made of Vitamin E polyethylene with a titanium coating is implanted into the patient’s acetabulum. A comprehensive range of sizes is offered to match most patients. Both components are designed to be permanently implanted to achieve reconstructive and functional hip joint replacement.
The PHR System is intended to offer benefits over total hip arthroplasties, such as bone conservation and restoration of more natural biomechanics, without a metal-on-metal articulating surface.
The IDE study has officially launched at seven esteemed clinical sites, with additional sites to be added in coming months.
MatOrtho Gains CE Mark for ReCerf Hip Resurfacing

MatOrtho received approval under the CE Mark for ReCerf Hip Resurfacing Arthroplasty (HRA).
ReCerf is reportedly the world’s first commercially available ceramic HRA, first approved by Australia’s Therapeutic Goods Administration in 2024. Since its initial use in 2018, over 1,600 patients have received the device. Patient-reported outcomes are highly positive, and the revision rate remains very low up to six years.
Made from BIOLOX delta ceramic, ReCerf offers an alternative to metal-on-metal bearings and preserves more of the patient’s natural bone.
Hip replacement has evolved significantly since the earliest attempts, which occurred in the late 19th century and employed materials like ivory and glass. How far we've come.
For 2024, hip replacement revenue reached $8.75 billion with growth of 4.5% vs. 2023. ORTHOWORLD estimates that growth arose from ongoing procedure recovery from prior...
Hip replacement has evolved significantly since the earliest attempts, which occurred in the late 19th century and employed materials like ivory and glass. How far we’ve come.
For 2024, hip replacement revenue reached $8.75 billion with growth of 4.5% vs. 2023. ORTHOWORLD estimates that growth arose from ongoing procedure recovery from prior years, as well as developing tailwinds. Here are four implant and technology developments that emerged in the past quarter to further advance modern total hip replacement.
First Clinical Use of Corin’s ApolloHipX
 ApolloHipX is the first total hip arthroplasty solution to combine dynamic 3D preoperative planning with real-time intraoperative assessment from standard fluoroscopy.
ApolloHipX is the first total hip arthroplasty solution to combine dynamic 3D preoperative planning with real-time intraoperative assessment from standard fluoroscopy.
ApolloHipX integrates Corin’s preoperative planning solution with intraoperative fluoroscopy via proprietary, automated 2D–3D registration software. By aligning a single 2D x-ray with the patient-specific 3D plan, the system provides a real-time 3D assessment of implant position, regardless of patient positioning or C-arm alignment. This streamlined approach to fluoroscopy-based THA reduces the number of intraoperative images required, minimizing surgical time and radiation exposure within the O.R.
Apollo was built as a connected surgical ecosystem — integrating AI-driven planning, intraoperative tools and cloud-based analytics to support more consistent, personalized orthopedic care. ApolloKnee, introduced in 2024, brought pre-resection balance assessment into the operating room with BalanceBot, enabling data-driven implant decisions before bone cuts are made. ApolloHipX now extends that same foundation to total hip replacement, offering real-time 3D validation within a streamlined, fluoroscopy-based workflow.
First Patient Treated with Alteon Short Tapered Wedge Hip

Exactech announced the first surgery using the new Alteon Short Tapered Wedge Hip Stem. Currently in limited launch across select U.S. sites, full U.S. release is slated for 4Q25.
Engineered to conserve distal bone, the new stem incorporates features designed to achieve immediate axial and rotational stability while reproducibly creating the desired leg length and offset.
Compared to previous generation wedge stems, the Alteon Tapered Wedge Stems intentionally grow at a smaller rate laterally and distally, especially in the smaller stem sizes. In addition, the Short Tapered Wedge is up to 25% shorter than standard Tapered Wedge stems.
JointMedica Launches Investigational Device Exemption Study for Polymotion Hip Resurfacing

The study aims to evaluate the system’s safety and clinical effectiveness in addressing the challenges faced by younger, more active patients.
Polymotion Hip Resurfacing conserves bone by removing less of the femoral head than traditional hip replacement, reshaping it to accommodate a metal cap that mirrors the natural anatomy. A cup made of Vitamin E polyethylene with a titanium coating is implanted into the patient’s acetabulum. A comprehensive range of sizes is offered to match most patients. Both components are designed to be permanently implanted to achieve reconstructive and functional hip joint replacement.
The PHR System is intended to offer benefits over total hip arthroplasties, such as bone conservation and restoration of more natural biomechanics, without a metal-on-metal articulating surface.
The IDE study has officially launched at seven esteemed clinical sites, with additional sites to be added in coming months.
MatOrtho Gains CE Mark for ReCerf Hip Resurfacing

MatOrtho received approval under the CE Mark for ReCerf Hip Resurfacing Arthroplasty (HRA).
ReCerf is reportedly the world’s first commercially available ceramic HRA, first approved by Australia’s Therapeutic Goods Administration in 2024. Since its initial use in 2018, over 1,600 patients have received the device. Patient-reported outcomes are highly positive, and the revision rate remains very low up to six years.
Made from BIOLOX delta ceramic, ReCerf offers an alternative to metal-on-metal bearings and preserves more of the patient’s natural bone.

You’ve reached your limit.
We’re glad you’re finding value in our content — and we’d love for you to keep going.
Subscribe now for unlimited access to orthopedic business intelligence.
JV
Julie Vetalice is ORTHOWORLD's Editorial Assistant. She has covered the orthopedic industry for over 20 years, having joined the company in 1999.