Copy to clipboard
Copy to clipboard 
Hip Innovation Technology (HIT) received FDA Investigational Device Exemption (IDE) approval to initiate a pivotal clinical study to further evaluate the company’s Reverse Hip Replacement System (Reverse HRS) for use in primary total hip arthroplasty (THA).
The clinical study objective is to evaluate the safety and effectiveness of the Reverse HRS in patients undergoing THA. Safety will be assessed through the collection of device-related adverse events and patient quality of life metrics. Effectiveness will be evaluated using clinical, radiologic and patient-reported outcomes.
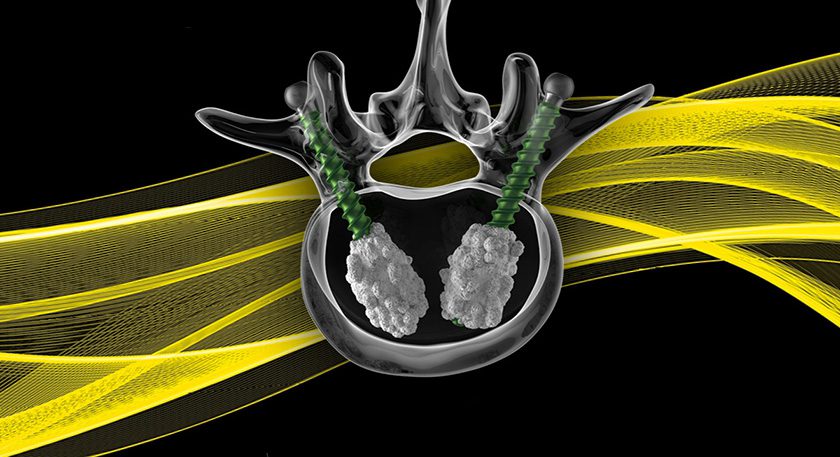
The Reverse HRS is a metal-on-polyethylene reverse geometry hip prosthesis designed to improve stability at extended ranges of motion and reduce the risk of dislocation. Like most conventional systems, Reverse HRS consists of a femoral stem, an acetabular cup and a cobalt-chrome ball that articulates within a polyethylene liner. Unlike existing total hip replacement systems, the ball is placed on a trunnion within the acetabular cup instead of the femoral stem, and the polyethylene liner is attached to a femoral cup, which then attaches to the femoral stem, as opposed to the polyethylene liner being attached to the acetabular cup.
“The Reverse HRS is a unique hip implant design that represents a significant advancement for patients requiring total hip arthroplasty,” said George Diamantoni, Hip Innovation Technology’s Co-Founder and Chief Executive Officer. “In our pivotal study we will further evaluate potential Reverse HRS patient benefits including hip stability at extended ranges of motion, reduced risk of device dislocation, and greater latitude for placement of hip components.”
Source: Hip Innovation Technology, LLC
Hip Innovation Technology (HIT) received FDA Investigational Device Exemption (IDE) approval to initiate a pivotal clinical study to further evaluate the company's Reverse Hip Replacement System (Reverse HRS) for use in primary total hip arthroplasty (THA).
The clinical study objective is to evaluate the safety and effectiveness of the Reverse...
Hip Innovation Technology (HIT) received FDA Investigational Device Exemption (IDE) approval to initiate a pivotal clinical study to further evaluate the company’s Reverse Hip Replacement System (Reverse HRS) for use in primary total hip arthroplasty (THA).
The clinical study objective is to evaluate the safety and effectiveness of the Reverse HRS in patients undergoing THA. Safety will be assessed through the collection of device-related adverse events and patient quality of life metrics. Effectiveness will be evaluated using clinical, radiologic and patient-reported outcomes.
The Reverse HRS is a metal-on-polyethylene reverse geometry hip prosthesis designed to improve stability at extended ranges of motion and reduce the risk of dislocation. Like most conventional systems, Reverse HRS consists of a femoral stem, an acetabular cup and a cobalt-chrome ball that articulates within a polyethylene liner. Unlike existing total hip replacement systems, the ball is placed on a trunnion within the acetabular cup instead of the femoral stem, and the polyethylene liner is attached to a femoral cup, which then attaches to the femoral stem, as opposed to the polyethylene liner being attached to the acetabular cup.
“The Reverse HRS is a unique hip implant design that represents a significant advancement for patients requiring total hip arthroplasty,” said George Diamantoni, Hip Innovation Technology’s Co-Founder and Chief Executive Officer. “In our pivotal study we will further evaluate potential Reverse HRS patient benefits including hip stability at extended ranges of motion, reduced risk of device dislocation, and greater latitude for placement of hip components.”
Source: Hip Innovation Technology, LLC

You’ve reached your limit.
We’re glad you’re finding value in our content — and we’d love for you to keep going.
Subscribe now for unlimited access to orthopedic business intelligence.
JV
Julie Vetalice is ORTHOWORLD's Editorial Assistant. She has covered the orthopedic industry for over 20 years, having joined the company in 1999.