
 Copy to clipboard
Copy to clipboard 
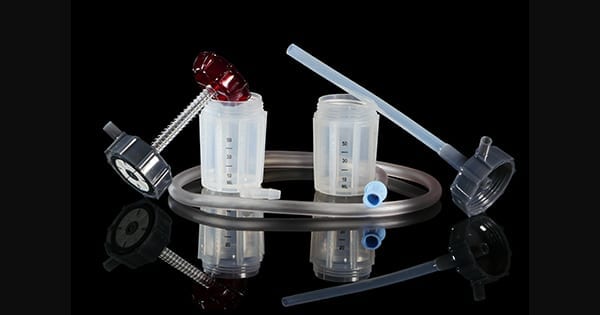
Hensler Surgical Technologies was granted approval under the CE Mark for the Hensler Bone Press™ (HBP).
Hensler Surgical brought the Hensler Bone Press to market in 2012. The HBP has indications for not just spinal surgery, but also orthopedic surgical cases to harvest autologous material whenever a high-speed drill is being operated.
HBP incorporates a clinically proven two-step technique to harvest high speed drilled autologous material during bony decompression such as laminectomies, vertebral corpectomies, orthopedic and foot & ankle surgeries. It is designed to be a high yield harvesting device with two 80 cc chambers.
“Harvesting drilled graft material is an arduous but necessary undertaking to improve fusion outcomes in a wide array of surgical procedures. We are proud to offer a device that procures this graft material quickly, allowing for the procurement and processing of this critical and valuable autologous resource into a moldable and malleable graft for use in fusion surgeries. I invented it simply out of the need in our own surgical cases, and it grew from there,” said Hensler Surgical CEO and inventor, Sean Hensler.
Hensler Surgical Technologies was granted approval under the CE Mark for the Hensler Bone Press™ (HBP).
Hensler Surgical brought the Hensler Bone Press to market in 2012. The HBP has indications for not just spinal surgery, but also orthopedic surgical cases to harvest autologous material whenever a high-speed drill is being operated.
...
Hensler Surgical Technologies was granted approval under the CE Mark for the Hensler Bone Press™ (HBP).
Hensler Surgical brought the Hensler Bone Press to market in 2012. The HBP has indications for not just spinal surgery, but also orthopedic surgical cases to harvest autologous material whenever a high-speed drill is being operated.
HBP incorporates a clinically proven two-step technique to harvest high speed drilled autologous material during bony decompression such as laminectomies, vertebral corpectomies, orthopedic and foot & ankle surgeries. It is designed to be a high yield harvesting device with two 80 cc chambers.
“Harvesting drilled graft material is an arduous but necessary undertaking to improve fusion outcomes in a wide array of surgical procedures. We are proud to offer a device that procures this graft material quickly, allowing for the procurement and processing of this critical and valuable autologous resource into a moldable and malleable graft for use in fusion surgeries. I invented it simply out of the need in our own surgical cases, and it grew from there,” said Hensler Surgical CEO and inventor, Sean Hensler.

You are out of free articles for this month
Subscribe as a Guest for $0 and unlock a total of 5 articles per month.
You are out of five articles for this month
Subscribe as an Executive Member for access to unlimited articles, THE ORTHOPAEDIC INDUSTRY ANNUAL REPORT and more.
JV
Julie Vetalice is ORTHOWORLD's Editorial Assistant. She has covered the orthopedic industry for over 20 years, having joined the company in 1999.







