
 Copy to clipboard
Copy to clipboard 
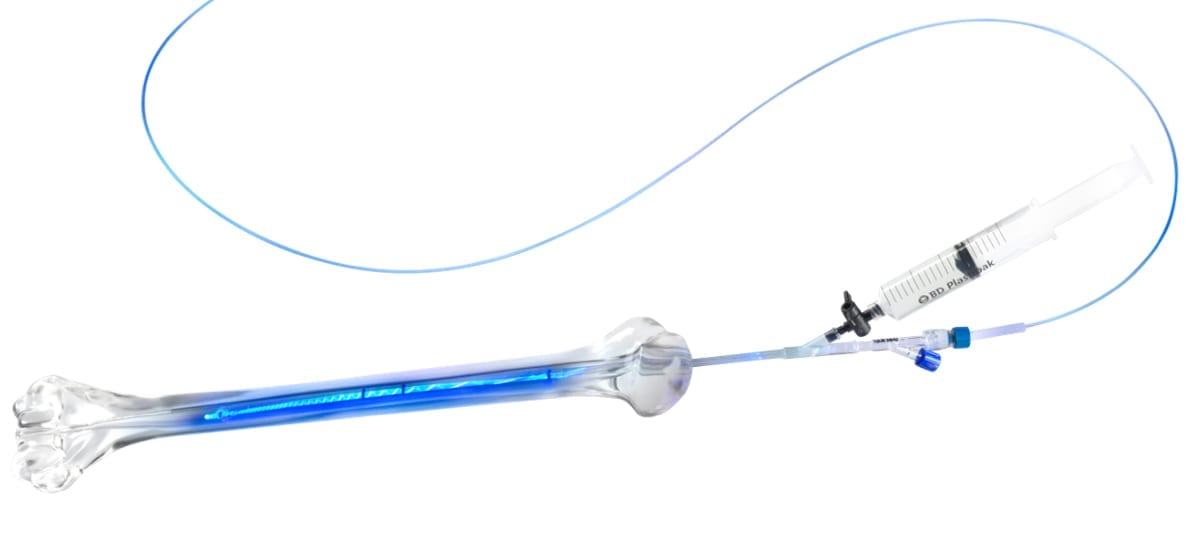
HealthpointCapital has claimed a majority stake in IlluminOss Medical, developer of the Photodynamic Bone Stabilization System that provides a minimally invasive approach for fracture repair and stabilization through a patient-specific intramedullary implant.
The system employs a light-curable liquid polymer, contained within an expandable balloon, to create a patient-conforming, rigid implant within the bone canal. The technology is particularly suited to treat the elderly patient population, as it enables increased stability for osteoporotic and compromised bone, as well as early mobilization. IlluminOss can be also used with cleared plates, screws and nails for supplemental fixation in compromised bone.
The IlluminOss system is CE Marked in Europe and FDA 510(k) cleared in the U.S. to treat a range of traumatic, fragility and pathological fractures. HealthpointCapital will partner with the IlluminOss team to drive growth and expand regulatory approvals for additional anatomical indications.
Commenting on the announcement, Mike Mogul, HealthpointCapital’s President, said, “IlluminOss has developed an ingenious solution for many challenging fracture indications. It can benefit a range of patients, including many of those treated conservatively today and help return them faster to normal activity.”
“We are convinced that our technology can change outcomes for elderly trauma patients,” said Robert Rabiner, Chief Technical Officer and Founder of IlluminOss. “This collaboration with HealthpointCapital will allow us to expand our patient base, finish our development, and accelerate commercialization.”
HealthpointCapital has claimed a majority stake in IlluminOss Medical, developer of the Photodynamic Bone Stabilization System that provides a minimally invasive approach for fracture repair and stabilization through a patient-specific intramedullary implant.
The system employs a light-curable liquid polymer, contained within an expandable...
HealthpointCapital has claimed a majority stake in IlluminOss Medical, developer of the Photodynamic Bone Stabilization System that provides a minimally invasive approach for fracture repair and stabilization through a patient-specific intramedullary implant.
The system employs a light-curable liquid polymer, contained within an expandable balloon, to create a patient-conforming, rigid implant within the bone canal. The technology is particularly suited to treat the elderly patient population, as it enables increased stability for osteoporotic and compromised bone, as well as early mobilization. IlluminOss can be also used with cleared plates, screws and nails for supplemental fixation in compromised bone.
The IlluminOss system is CE Marked in Europe and FDA 510(k) cleared in the U.S. to treat a range of traumatic, fragility and pathological fractures. HealthpointCapital will partner with the IlluminOss team to drive growth and expand regulatory approvals for additional anatomical indications.
Commenting on the announcement, Mike Mogul, HealthpointCapital’s President, said, “IlluminOss has developed an ingenious solution for many challenging fracture indications. It can benefit a range of patients, including many of those treated conservatively today and help return them faster to normal activity.”
“We are convinced that our technology can change outcomes for elderly trauma patients,” said Robert Rabiner, Chief Technical Officer and Founder of IlluminOss. “This collaboration with HealthpointCapital will allow us to expand our patient base, finish our development, and accelerate commercialization.”

You are out of free articles for this month
Subscribe as a Guest for $0 and unlock a total of 5 articles per month.
You are out of five articles for this month
Subscribe as an Executive Member for access to unlimited articles, THE ORTHOPAEDIC INDUSTRY ANNUAL REPORT and more.
JV
Julie Vetalice is ORTHOWORLD's Editorial Assistant. She has covered the orthopedic industry for over 20 years, having joined the company in 1999.







