
 Copy to clipboard
Copy to clipboard 
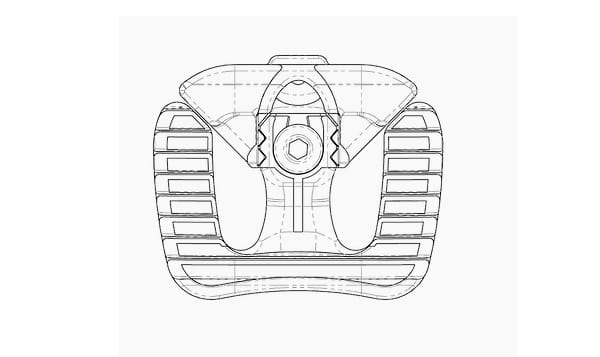
HD LifeSciences received FDA 510(k) clearance for its Hive™ ALIF Standalone Lumbar Interbody Fusion System. The implant’s modular anterior portion allows surgeon choice and flexibility to use integrated zero-profile fixation or anterior plating. A patent-pending mechanism allows the surgeon to adjust the footprint of the device before implantation.
Hive ALIF features a range of implant configurations with multiple width, height, depth and lordotic choices.
HD Lifesciences has developed Hive Soft Titanium® technology. The Hive lattice with NanoHive® Surface Technology is designed to provide ideal elastic modulus, diagnostic imaging and osteogenic properties.
“This system is the next step forward for HD’s Hive implants,” says David Rattigan, Vice President of Sales for HD LifeSciences. “We are proud to offer this differentiated implant technology to our surgeon users.”
HD LifeSciences received FDA 510(k) clearance for its Hive™ ALIF Standalone Lumbar Interbody Fusion System. The implant's modular anterior portion allows surgeon choice and flexibility to use integrated zero-profile fixation or anterior plating. A patent-pending mechanism allows the surgeon to adjust the footprint of the device before...
HD LifeSciences received FDA 510(k) clearance for its Hive™ ALIF Standalone Lumbar Interbody Fusion System. The implant’s modular anterior portion allows surgeon choice and flexibility to use integrated zero-profile fixation or anterior plating. A patent-pending mechanism allows the surgeon to adjust the footprint of the device before implantation.
Hive ALIF features a range of implant configurations with multiple width, height, depth and lordotic choices.
HD Lifesciences has developed Hive Soft Titanium® technology. The Hive lattice with NanoHive® Surface Technology is designed to provide ideal elastic modulus, diagnostic imaging and osteogenic properties.
“This system is the next step forward for HD’s Hive implants,” says David Rattigan, Vice President of Sales for HD LifeSciences. “We are proud to offer this differentiated implant technology to our surgeon users.”

You are out of free articles for this month
Subscribe as a Guest for $0 and unlock a total of 5 articles per month.
You are out of five articles for this month
Subscribe as an Executive Member for access to unlimited articles, THE ORTHOPAEDIC INDUSTRY ANNUAL REPORT and more.
JV
Julie Vetalice is ORTHOWORLD's Editorial Assistant. She has covered the orthopedic industry for over 20 years, having joined the company in 1999.







