

 Copy to clipboard
Copy to clipboard 
In observing the lineup of spine revenue over $100 million halfway through the year, as shown in Exhibit 1, we note that NuVasive stands at the forefront of growth while pure-play companies like Globus and K2M are outperforming their diversified peers in the field. (Please note, the numbers below exclude orthobiologics.)
Exhibit 1
Spine Segment Sales, 1H16 vs. 1H15 ($MM)
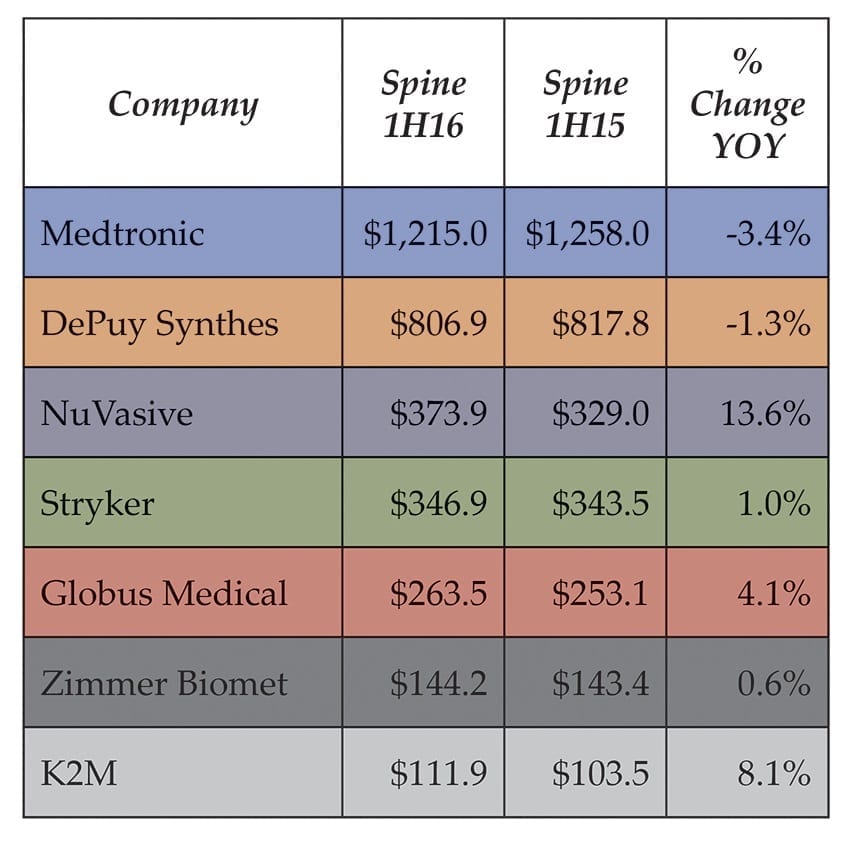
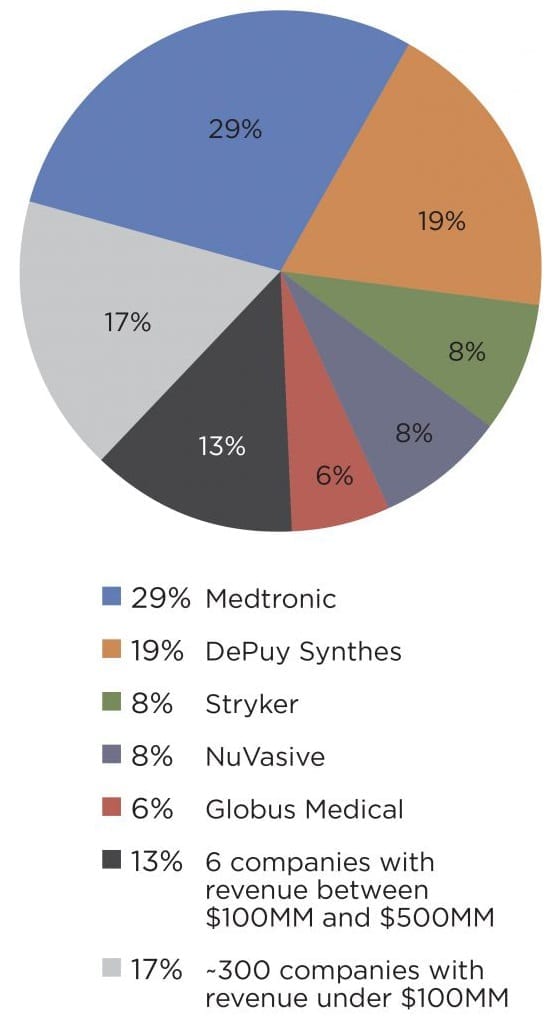
Exhibit 2
Spine Market Share: Largest Players and All Others, 2015
What actions have these companies undertaken to complement their current offerings, broaden their portfolios and grow their share of the market before the end of 2016? Let’s look at what’s gone down in the first half.
MEDTRONIC
- Launched an investment and development agreement with Mazor to shore up robotic surgical navigation for spine procedures
- Received FDA Premarket Approval for Prestige LP to treat cervical disc disease at two adjacent levels
- Received FDA approval of INFUSE Bone Graft for new indications of oblique lateral and anterior lumbar interbody fusion and launched new products for OLIF
- Committed to make investments in instrument sets and speed execution of product launches that encompass a whole procedural solution (vs. just piecemeal product releases); also taking advantage of sales synergies offered by navigation and O-arm systems
DEPUY SYNTHES
- Per intel gathered at the Business Review Day, pipeline plan through 2018 has seven products that pertain to spine, including a navigation system and expandable interbody cage
- Plans to close key portfolio gaps with targeted M&A, upgrade key platforms and simplify the current portfolio (suggests brand reduction)
- Also noted at Review Day, spine is important to cross-selling efforts in other businesses, like Surgical, and JNJ’s strength in scale will be of extreme value for the spine segment as regulatory challenges ex-U.S. just grow and grow: JNJ’s scale should lend needed muscle to gain regulatory wins
NUVASIVE
- Acquired Mega Surgical, its exclusive distributor in Brazil, as well as Biotronic NeuroNetwork and Ellipse Technologies—supplementary to its existing neurophysiological monitoring and scoliosis lines, respectively
- Secured a CMS add-on payment for MAGEC magnetically-controlled growth rods, cleared by FDA for the treatment of pediatric spinal deformity
- Gaining momentum with the iGA (Integrated Global Alignment) system, which drives pull-through of spinal hardware sales (specifically, leadership has noted progress in securing contracts with U.S. healthcare systems for bundled offerings that include the NVM5 nerve monitoring system and iGA)
STRYKER
- Acquisition of Safewire brought products that support minimally invasive procedures; the company also snapped up BD’s vertebral augmentation portfolio, which was cleared via FDA 510(k) under CareFusion
- Reporting good demand for 3D-printed Tritanium Posterior Lumbar Cages
- Co-promoting EU spine business with the EOS imaging platform to existing and prospective customers in the U.K.
- Launched what is reportedly the only ACDF device to offer uniform compression across the interbody space: the Aero®-C Cervical Stability System, which also offers a less disruptive approach vs. traditional screw-based ACDF
GLOBUS MEDICAL
- Took steps to double its ex-U.S. revenue by picking up Alphatec Spine’s international business, gaining immediate access to Brazil and Japan, as well as sales operations in Italy and the U.K.
- Starting to hire reps for navigation/robotics division will start within 2016
ZIMMER BIOMET
- Acquired LDR and Medtech
- LDR’s Mobi-C cervical disc has FDA approval for 2-level applications, and transaction brings cross-selling of portfolios that also address minimally invasive procedures and complex spine, coupled with biologics
- Medtech’s ROSA™ Spine robotic-assistive device for minimally invasive surgery received CE Mark approval in 2014 and FDA 510(k) clearance in 1Q16; 100 spinal surgeries have been successfully performed with the system
K2M
- Entered the cervical disc market ex-U.S. with CE Mark approval gained for the RHINE Cervical Disc
- Has affirmed that it intends to target ex-U.S. markets initially to gauge performance and interest in RHINE
- Also going the 3D route: received FDA 510(k) clearances to market CASCADIA™ Cervical and CASCADIA AN Lordotic Oblique Interbody Systems, featuring Lamellar 3D-printed Titanium Technology™
In observing the lineup of spine revenue over $100 million halfway through the year, as shown in Exhibit 1, we note that NuVasive stands at the forefront of growth while pure-play companies like Globus and K2M are outperforming their diversified peers in the field. (Please note, the numbers below exclude orthobiologics.)
Exhibit 1
Spine Segment...
In observing the lineup of spine revenue over $100 million halfway through the year, as shown in Exhibit 1, we note that NuVasive stands at the forefront of growth while pure-play companies like Globus and K2M are outperforming their diversified peers in the field. (Please note, the numbers below exclude orthobiologics.)
Exhibit 1
Spine Segment Sales, 1H16 vs. 1H15 ($MM)

Exhibit 2
Spine Market Share: Largest Players and All Others, 2015

What actions have these companies undertaken to complement their current offerings, broaden their portfolios and grow their share of the market before the end of 2016? Let’s look at what’s gone down in the first half.
MEDTRONIC
- Launched an investment and development agreement with Mazor to shore up robotic surgical navigation for spine procedures
- Received FDA Premarket Approval for Prestige LP to treat cervical disc disease at two adjacent levels
- Received FDA approval of INFUSE Bone Graft for new indications of oblique lateral and anterior lumbar interbody fusion and launched new products for OLIF
- Committed to make investments in instrument sets and speed execution of product launches that encompass a whole procedural solution (vs. just piecemeal product releases); also taking advantage of sales synergies offered by navigation and O-arm systems
DEPUY SYNTHES
- Per intel gathered at the Business Review Day, pipeline plan through 2018 has seven products that pertain to spine, including a navigation system and expandable interbody cage
- Plans to close key portfolio gaps with targeted M&A, upgrade key platforms and simplify the current portfolio (suggests brand reduction)
- Also noted at Review Day, spine is important to cross-selling efforts in other businesses, like Surgical, and JNJ’s strength in scale will be of extreme value for the spine segment as regulatory challenges ex-U.S. just grow and grow: JNJ’s scale should lend needed muscle to gain regulatory wins
NUVASIVE
- Acquired Mega Surgical, its exclusive distributor in Brazil, as well as Biotronic NeuroNetwork and Ellipse Technologies—supplementary to its existing neurophysiological monitoring and scoliosis lines, respectively
- Secured a CMS add-on payment for MAGEC magnetically-controlled growth rods, cleared by FDA for the treatment of pediatric spinal deformity
- Gaining momentum with the iGA (Integrated Global Alignment) system, which drives pull-through of spinal hardware sales (specifically, leadership has noted progress in securing contracts with U.S. healthcare systems for bundled offerings that include the NVM5 nerve monitoring system and iGA)
STRYKER
- Acquisition of Safewire brought products that support minimally invasive procedures; the company also snapped up BD’s vertebral augmentation portfolio, which was cleared via FDA 510(k) under CareFusion
- Reporting good demand for 3D-printed Tritanium Posterior Lumbar Cages
- Co-promoting EU spine business with the EOS imaging platform to existing and prospective customers in the U.K.
- Launched what is reportedly the only ACDF device to offer uniform compression across the interbody space: the Aero®-C Cervical Stability System, which also offers a less disruptive approach vs. traditional screw-based ACDF
GLOBUS MEDICAL
- Took steps to double its ex-U.S. revenue by picking up Alphatec Spine’s international business, gaining immediate access to Brazil and Japan, as well as sales operations in Italy and the U.K.
- Starting to hire reps for navigation/robotics division will start within 2016
ZIMMER BIOMET
- Acquired LDR and Medtech
- LDR’s Mobi-C cervical disc has FDA approval for 2-level applications, and transaction brings cross-selling of portfolios that also address minimally invasive procedures and complex spine, coupled with biologics
- Medtech’s ROSA™ Spine robotic-assistive device for minimally invasive surgery received CE Mark approval in 2014 and FDA 510(k) clearance in 1Q16; 100 spinal surgeries have been successfully performed with the system
K2M
- Entered the cervical disc market ex-U.S. with CE Mark approval gained for the RHINE Cervical Disc
- Has affirmed that it intends to target ex-U.S. markets initially to gauge performance and interest in RHINE
- Also going the 3D route: received FDA 510(k) clearances to market CASCADIA™ Cervical and CASCADIA AN Lordotic Oblique Interbody Systems, featuring Lamellar 3D-printed Titanium Technology™

You are out of free articles for this month
Subscribe as a Guest for $0 and unlock a total of 5 articles per month.
You are out of five articles for this month
Subscribe as an Executive Member for access to unlimited articles, THE ORTHOPAEDIC INDUSTRY ANNUAL REPORT and more.
JV
Julie Vetalice is ORTHOWORLD's Editorial Assistant. She has covered the orthopedic industry for over 20 years, having joined the company in 1999.







