
 Copy to clipboard
Copy to clipboard 
Graftys achieved CE recertification in Europe and the certification of its Quality Management System in Australia, Brazil, Canada and the U.S. for the design, manufacture and distribution of its sterile calcium phosphate resorbable bone void fillers and related delivery systems.
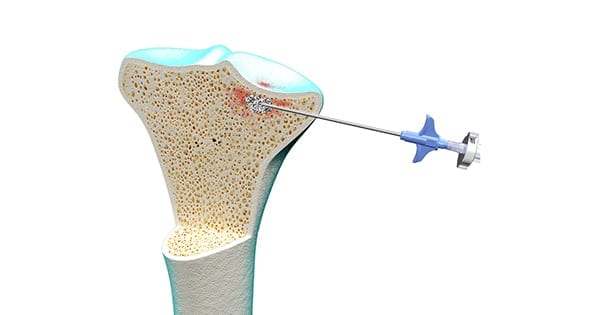
The company’s products include high viscosity Graftys QuickSet (pictured above) and lower viscosity Graftys® HBS to facilitate remote injection in close cavities. The latter is only available in Brazil.
“By achieving the MDSAP certification and the CE recertification of our bone cements,
we continue our commitment to reach the highest Quality Assurance standards and
to strictly comply with local laws and regulatory requirements in all jurisdictions we are
operating in, including Europe, North and South America, for the benefits of our
partners, our surgeons and most importantly our patients,” said Enrico
Bastianelli, M.D., MBA, Chief Executive Officer of Graftys.
Graftys achieved CE recertification in Europe and the certification of its Quality Management System in Australia, Brazil, Canada and the U.S. for the design, manufacture and distribution of its sterile calcium phosphate resorbable bone void fillers and related delivery systems.
The company's products include high viscosity Graftys QuickSet...
Graftys achieved CE recertification in Europe and the certification of its Quality Management System in Australia, Brazil, Canada and the U.S. for the design, manufacture and distribution of its sterile calcium phosphate resorbable bone void fillers and related delivery systems.
The company’s products include high viscosity Graftys QuickSet (pictured above) and lower viscosity Graftys® HBS to facilitate remote injection in close cavities. The latter is only available in Brazil.
“By achieving the MDSAP certification and the CE recertification of our bone cements,
we continue our commitment to reach the highest Quality Assurance standards and
to strictly comply with local laws and regulatory requirements in all jurisdictions we are
operating in, including Europe, North and South America, for the benefits of our
partners, our surgeons and most importantly our patients,” said Enrico
Bastianelli, M.D., MBA, Chief Executive Officer of Graftys.

You are out of free articles for this month
Subscribe as a Guest for $0 and unlock a total of 5 articles per month.
You are out of five articles for this month
Subscribe as an Executive Member for access to unlimited articles, THE ORTHOPAEDIC INDUSTRY ANNUAL REPORT and more.
JV
Julie Vetalice is ORTHOWORLD's Editorial Assistant. She has covered the orthopedic industry for over 20 years, having joined the company in 1999.







