Copy to clipboard
Copy to clipboard 
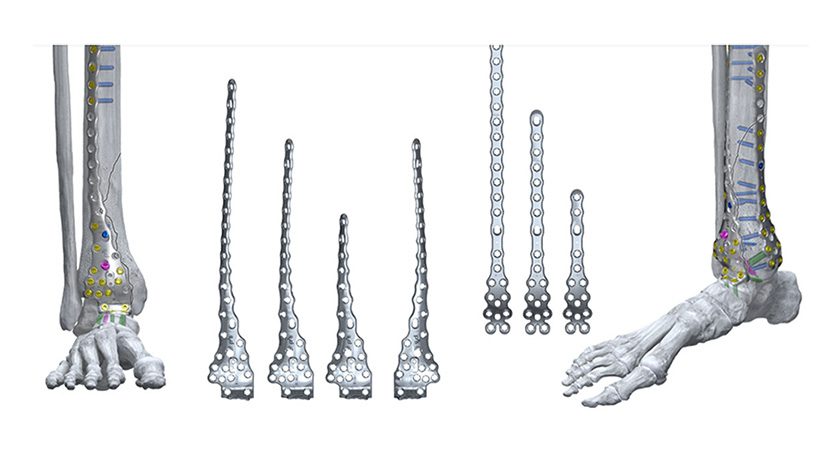
We are proud to announce that the GMReis Fibula Nail is now FDA 510(k) cleared for marketing in the U.S.
With over eight years of clinical follow-up, the GMReis Fibula Nail offers a modern solution for specific applications where a minimally invasive approach is critical:
- Elderly patients
- Patients with poor skin quality
- Other selected indications where traditional plating may not be optimal
GMReis is exhibiting at OMTEC from June 17-19. Visit them at booth 715.
Source: GMReis
We are proud to announce that the GMReis Fibula Nail is now FDA 510(k) cleared for marketing in the U.S.
With over eight years of clinical follow-up, the GMReis Fibula Nail offers a modern solution for specific applications where a minimally invasive approach is critical:
Elderly patients
Patients with poor skin...
We are proud to announce that the GMReis Fibula Nail is now FDA 510(k) cleared for marketing in the U.S.
With over eight years of clinical follow-up, the GMReis Fibula Nail offers a modern solution for specific applications where a minimally invasive approach is critical:
- Elderly patients
- Patients with poor skin quality
- Other selected indications where traditional plating may not be optimal
GMReis is exhibiting at OMTEC from June 17-19. Visit them at booth 715.
Source: GMReis

You are out of free articles for this month
Subscribe as a Guest for $0 and unlock a total of 5 articles per month.
You are out of five articles for this month
Subscribe as an Executive Member for access to unlimited articles, THE ORTHOPAEDIC INDUSTRY ANNUAL REPORT and more.
JV
Julie Vetalice is ORTHOWORLD's Editorial Assistant. She has covered the orthopedic industry for over 20 years, having joined the company in 1999.