
 Copy to clipboard
Copy to clipboard 
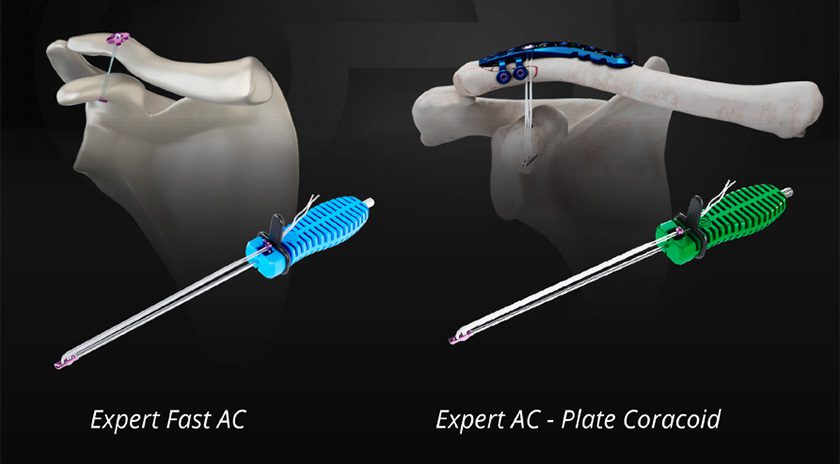
GMReis received FDA 510(k) clearance to market the Expert AC – Knotless flexible fixation device for acute or chronic acromioclavicular joint reduction and stabilization.
The clearance also includes the Expert AC – Plate – Coracoid, indicated for secondary fixation of the coracoid associated with distal clavicular plate in cases of fracture. This model has a dedicated design that will interface properly with a clavicle plate autocompression hole.
Both models are preloaded onto a unique inserter to facilitate implantation under fluoroscopy via open procedure, avoiding the use of scope or access beneath the coracoid.
Source: GMReis
GMReis received FDA 510(k) clearance to market the Expert AC - Knotless flexible fixation device for acute or chronic acromioclavicular joint reduction and stabilization.
The clearance also includes the Expert AC - Plate - Coracoid, indicated for secondary fixation of the coracoid associated with distal clavicular plate in cases of fracture....
GMReis received FDA 510(k) clearance to market the Expert AC – Knotless flexible fixation device for acute or chronic acromioclavicular joint reduction and stabilization.
The clearance also includes the Expert AC – Plate – Coracoid, indicated for secondary fixation of the coracoid associated with distal clavicular plate in cases of fracture. This model has a dedicated design that will interface properly with a clavicle plate autocompression hole.
Both models are preloaded onto a unique inserter to facilitate implantation under fluoroscopy via open procedure, avoiding the use of scope or access beneath the coracoid.
Source: GMReis

You are out of free articles for this month
Subscribe as a Guest for $0 and unlock a total of 5 articles per month.
You are out of five articles for this month
Subscribe as an Executive Member for access to unlimited articles, THE ORTHOPAEDIC INDUSTRY ANNUAL REPORT and more.
JV
Julie Vetalice is ORTHOWORLD's Editorial Assistant. She has covered the orthopedic industry for over 20 years, having joined the company in 1999.







