Copy to clipboard
Copy to clipboard 
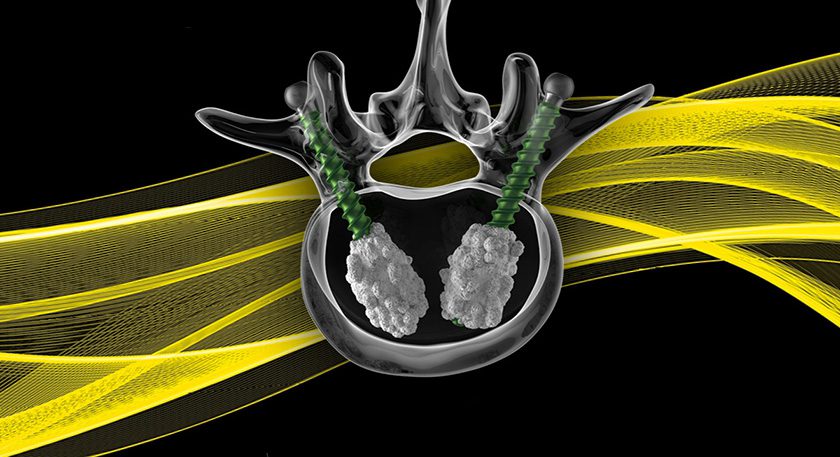
Globus Medical (GMED) received a letter from FDA, indicating that the company had not sufficiently addressed questions on its 510(k) submission for the ExcelsiusGPS™ robotic guidance and navigation system. GMED will expedite a revised filing, and no longer expects to receive FDA clearance in 2Q17.
Globus reiterated its full-year 2017 revenue guidance of US $625.0MM.
GMED announced its acquisition of Excelsius at the start of 2014, showcased the system at EuroSpine and NASS and filed for FDA clearance in the last quarter of 2016. CE Mark approval has been secured as of 1Q17.
Having spoken with company leadership, analysts note that FDA requested more data on the technology’s features, which GMED has at hand. Clearance is still expected within the year.
Sources: Form 8-K for Globus Medical, Inc., SEC.gov; ORTHOWORLD Inc.
Globus Medical (GMED) received a letter from FDA, indicating that the company had not sufficiently addressed questions on its 510(k) submission for the ExcelsiusGPS™ robotic guidance and navigation system. GMED will expedite a revised filing, and no longer expects to receive FDA clearance in 2Q17.
Globus reiterated its full-year 2017 revenue...
Globus Medical (GMED) received a letter from FDA, indicating that the company had not sufficiently addressed questions on its 510(k) submission for the ExcelsiusGPS™ robotic guidance and navigation system. GMED will expedite a revised filing, and no longer expects to receive FDA clearance in 2Q17.
Globus reiterated its full-year 2017 revenue guidance of US $625.0MM.
GMED announced its acquisition of Excelsius at the start of 2014, showcased the system at EuroSpine and NASS and filed for FDA clearance in the last quarter of 2016. CE Mark approval has been secured as of 1Q17.
Having spoken with company leadership, analysts note that FDA requested more data on the technology’s features, which GMED has at hand. Clearance is still expected within the year.
Sources: Form 8-K for Globus Medical, Inc., SEC.gov; ORTHOWORLD Inc.

You’ve reached your limit.
We’re glad you’re finding value in our content — and we’d love for you to keep going.
Subscribe now for unlimited access to orthopedic business intelligence.
JV
Julie Vetalice is ORTHOWORLD's Editorial Assistant. She has covered the orthopedic industry for over 20 years, having joined the company in 1999.