
 Copy to clipboard
Copy to clipboard 
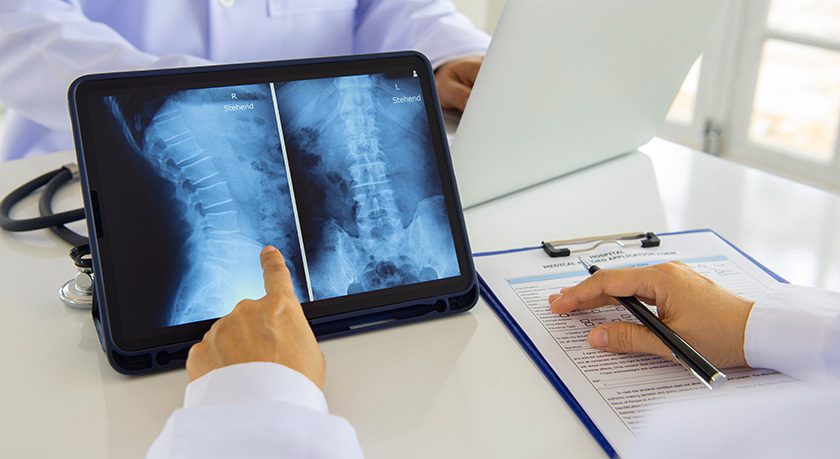
Globus Medical commenced commercial launch of the ADIRA XLIF Plate System, the company’s first product launch compatible across its expanded lateral interbody portfolio.
Procedurally integrated throughout the Globus Medical lateral portfolio, ADIRA plates are designed to rigidly thread into RISE-L, Modulus XLIF, Hedron L, Cohere XLIF, TransContinental and CoRoent XLIF interbody spacers, to help reduce the risk of spacer migration while accommodating varied patient anatomy and surgeon preferences.
ADIRA plates may be assembled to lateral lumbar interbody fusion devices with an alignment screw to create an ADIRA Plate/Spacer assembly, to provide structural stability in skeletally mature individuals following discectomy. The plate/spacer assembly is used with bone screws and/or lateral anchors.
ADIRA 2-Hole and 4-Hole Plates, when used with screws only, are intended for use in the treatment of thoracolumbar spinal instability as a result of fracture, tumor, degenerative disc disease, scoliosis, kyphosis, lordosis, spinal stenosis or failed previous spinal surgery.
The ADIRA 1-Hole Plate is intended to stabilize allograft or autograft at one level, aiding in spinal fusion and to provide temporary stabilization and augment development of a spinal fusion. It may be used alone or with other anterior, lateral, anterolateral or posterior spinal systems. The device is not intended for load bearing applications.
ADIRA Plates may be assembled to a lateral lumbar interbody fusion device to create a plate-spacer assembly. When assembled to HEDRON L, TransContinental or RISE-L Spacers, the plate/spacer assembly is indicated for use at one or more levels of the lumbosacral spine, as an adjunct to fusion in patients with degenerative disc disease, disc herniation, spondylolisthesis, deformity, spinal stenosis and failed previous fusion. When ADIRA Plates are assembled to Modulus XLIF, CoRoent or Cohere XLIF Spacers, the plate/spacer assembly takes on the indications of the interbody device.
“This launch represents an important milestone in our integration roadmap,” said David Hole, president of Spine at Globus Medical. “Through the system’s ability to be paired with our industry-leading Globus and NuVasive interbody spacers, ADIRA demonstrates our continued commitment to accelerated procedural innovation in lateral spine surgery.”
Source: Globus Medical, Inc.
Globus Medical commenced commercial launch of the ADIRA XLIF Plate System, the company’s first product launch compatible across its expanded lateral interbody portfolio.
Procedurally integrated throughout the Globus Medical lateral portfolio, ADIRA plates are designed to rigidly thread into RISE-L, Modulus XLIF, Hedron L, Cohere XLIF,...
Globus Medical commenced commercial launch of the ADIRA XLIF Plate System, the company’s first product launch compatible across its expanded lateral interbody portfolio.
Procedurally integrated throughout the Globus Medical lateral portfolio, ADIRA plates are designed to rigidly thread into RISE-L, Modulus XLIF, Hedron L, Cohere XLIF, TransContinental and CoRoent XLIF interbody spacers, to help reduce the risk of spacer migration while accommodating varied patient anatomy and surgeon preferences.
ADIRA plates may be assembled to lateral lumbar interbody fusion devices with an alignment screw to create an ADIRA Plate/Spacer assembly, to provide structural stability in skeletally mature individuals following discectomy. The plate/spacer assembly is used with bone screws and/or lateral anchors.
ADIRA 2-Hole and 4-Hole Plates, when used with screws only, are intended for use in the treatment of thoracolumbar spinal instability as a result of fracture, tumor, degenerative disc disease, scoliosis, kyphosis, lordosis, spinal stenosis or failed previous spinal surgery.
The ADIRA 1-Hole Plate is intended to stabilize allograft or autograft at one level, aiding in spinal fusion and to provide temporary stabilization and augment development of a spinal fusion. It may be used alone or with other anterior, lateral, anterolateral or posterior spinal systems. The device is not intended for load bearing applications.
ADIRA Plates may be assembled to a lateral lumbar interbody fusion device to create a plate-spacer assembly. When assembled to HEDRON L, TransContinental or RISE-L Spacers, the plate/spacer assembly is indicated for use at one or more levels of the lumbosacral spine, as an adjunct to fusion in patients with degenerative disc disease, disc herniation, spondylolisthesis, deformity, spinal stenosis and failed previous fusion. When ADIRA Plates are assembled to Modulus XLIF, CoRoent or Cohere XLIF Spacers, the plate/spacer assembly takes on the indications of the interbody device.
“This launch represents an important milestone in our integration roadmap,” said David Hole, president of Spine at Globus Medical. “Through the system’s ability to be paired with our industry-leading Globus and NuVasive interbody spacers, ADIRA demonstrates our continued commitment to accelerated procedural innovation in lateral spine surgery.”
Source: Globus Medical, Inc.

You are out of free articles for this month
Subscribe as a Guest for $0 and unlock a total of 5 articles per month.
You are out of five articles for this month
Subscribe as an Executive Member for access to unlimited articles, THE ORTHOPAEDIC INDUSTRY ANNUAL REPORT and more.
JV
Julie Vetalice is ORTHOWORLD's Editorial Assistant. She has covered the orthopedic industry for over 20 years, having joined the company in 1999.







