
 Copy to clipboard
Copy to clipboard 
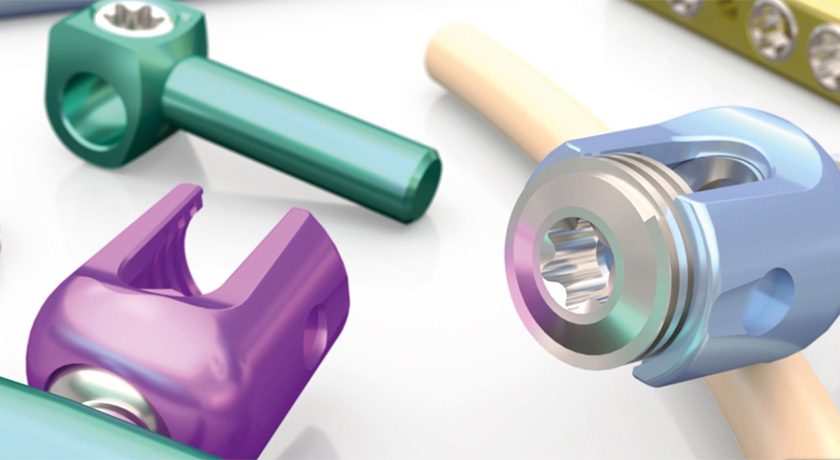
Spineway commenced the accelerated rollout of its range of VEOS implants for intervertebral fixation by posterior approach into new markets.
This technology has received CE/MD marking and FDA 510(k) clearance. Spineway is rolling out sales of its VEOS system in France, via direct sales to healthcare facilities, as well as in Europe and the export market through a network of importer-distributors.
In Spain, the distribution of the VEOS range has been entrusted to one of the major players in the distribution of orthopedic and spinal implants, and the first implants were performed on patients in hospitals in Barcelona earlier this month.
Further, the Spineway Group has received approvals from health authorities for the VEOS range to be marketed in Colombia and Indonesia.
Entry into these new markets should contribute to sales growth for this range in 2024 and strengthen the company’s positions in Latin America and Asia.
Source: Spineway
Spineway commenced the accelerated rollout of its range of VEOS implants for intervertebral fixation by posterior approach into new markets.
This technology has received CE/MD marking and FDA 510(k) clearance. Spineway is rolling out sales of its VEOS system in France, via direct sales to healthcare facilities, as well as in Europe and the...
Spineway commenced the accelerated rollout of its range of VEOS implants for intervertebral fixation by posterior approach into new markets.
This technology has received CE/MD marking and FDA 510(k) clearance. Spineway is rolling out sales of its VEOS system in France, via direct sales to healthcare facilities, as well as in Europe and the export market through a network of importer-distributors.
In Spain, the distribution of the VEOS range has been entrusted to one of the major players in the distribution of orthopedic and spinal implants, and the first implants were performed on patients in hospitals in Barcelona earlier this month.
Further, the Spineway Group has received approvals from health authorities for the VEOS range to be marketed in Colombia and Indonesia.
Entry into these new markets should contribute to sales growth for this range in 2024 and strengthen the company’s positions in Latin America and Asia.
Source: Spineway

You are out of free articles for this month
Subscribe as a Guest for $0 and unlock a total of 5 articles per month.
You are out of five articles for this month
Subscribe as an Executive Member for access to unlimited articles, THE ORTHOPAEDIC INDUSTRY ANNUAL REPORT and more.
JV
Julie Vetalice is ORTHOWORLD's Editorial Assistant. She has covered the orthopedic industry for over 20 years, having joined the company in 1999.







