
 Copy to clipboard
Copy to clipboard 
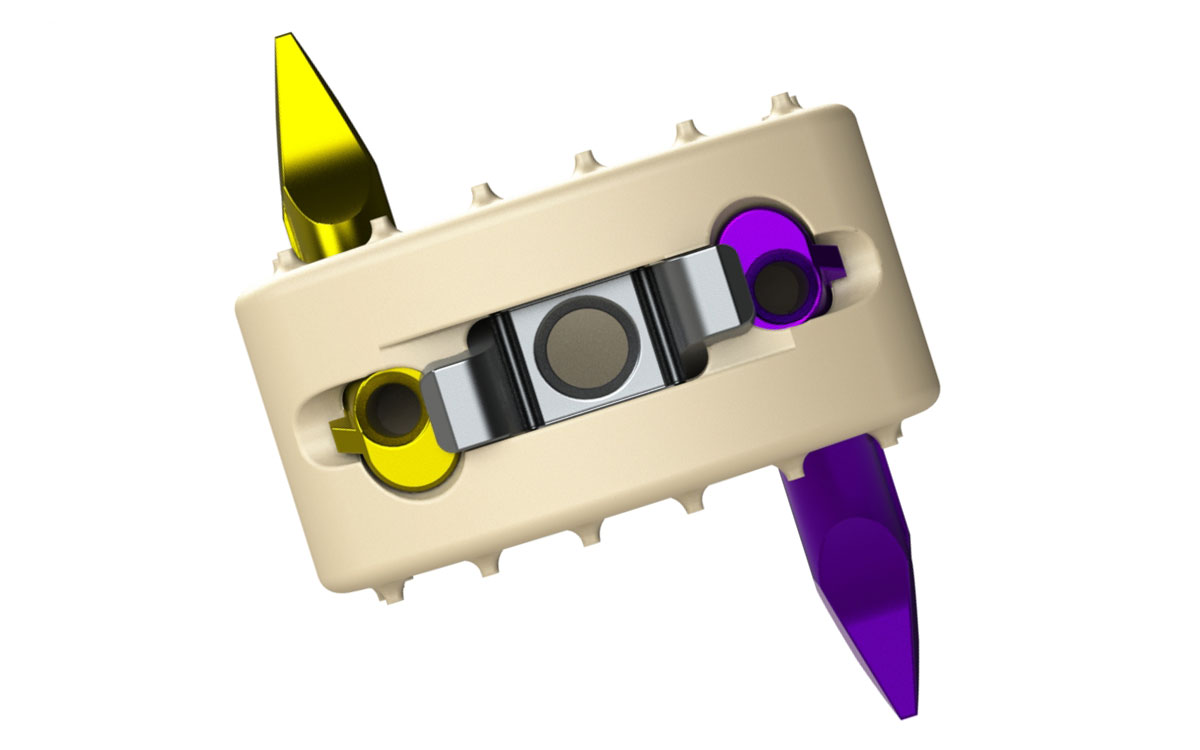 Genesys Spine received FDA 510(k) clearance and subsequently launched the AIS-C Stand-Alone interbody system for the cervical spine. The direct-anterior system is non-screw based and zero-profile, according to the company.
Genesys Spine received FDA 510(k) clearance and subsequently launched the AIS-C Stand-Alone interbody system for the cervical spine. The direct-anterior system is non-screw based and zero-profile, according to the company.
AIS-C Stand-Alone system also features a zero-step locking mechanism, a feature that provides visual confirmation of engagement, but allows for anchor removal when desired. Interbodies are offered in small (14mm wide x 12mm long) and large (17mm wide x 14mm long) footprints.
Genesys Spine’s flagship product is the TiLock Pedicle Screw System, which in 2010 was the company’s first 510(k) approval.
Sources: Genesys Spine; image courtesy of Genesys Spine
Genesys Spine received FDA 510(k) clearance and subsequently launched the AIS-C Stand-Alone interbody system for the cervical spine. The direct-anterior system is non-screw based and zero-profile, according to the company.
AIS-C Stand-Alone system also features a zero-step locking mechanism, a feature that provides visual confirmation of...
 Genesys Spine received FDA 510(k) clearance and subsequently launched the AIS-C Stand-Alone interbody system for the cervical spine. The direct-anterior system is non-screw based and zero-profile, according to the company.
Genesys Spine received FDA 510(k) clearance and subsequently launched the AIS-C Stand-Alone interbody system for the cervical spine. The direct-anterior system is non-screw based and zero-profile, according to the company.
AIS-C Stand-Alone system also features a zero-step locking mechanism, a feature that provides visual confirmation of engagement, but allows for anchor removal when desired. Interbodies are offered in small (14mm wide x 12mm long) and large (17mm wide x 14mm long) footprints.
Genesys Spine’s flagship product is the TiLock Pedicle Screw System, which in 2010 was the company’s first 510(k) approval.
Sources: Genesys Spine; image courtesy of Genesys Spine

You’ve reached your limit.
We’re glad you’re finding value in our content — and we’d love for you to keep going.
Subscribe now for unlimited access to orthopedic business intelligence.
JV
Julie Vetalice is ORTHOWORLD's Editorial Assistant. She has covered the orthopedic industry for over 20 years, having joined the company in 1999.







