
 Copy to clipboard
Copy to clipboard 
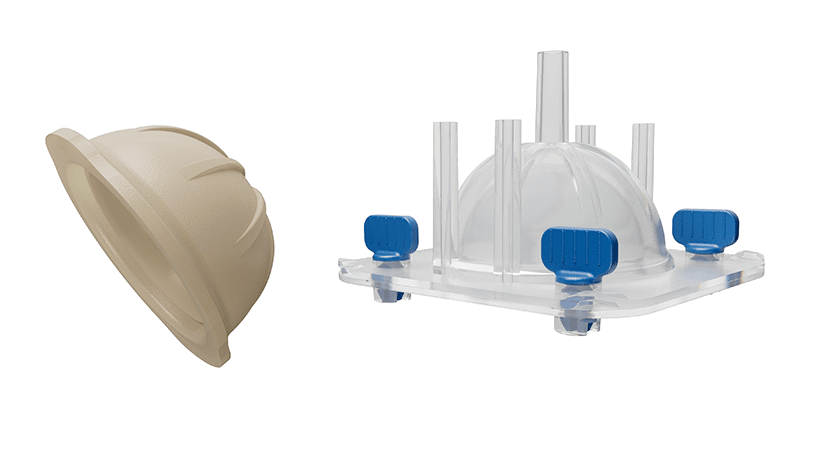
G-21 received FDA 510(k) clearance to market the SpaceFlex Acetabular Cup. The temporary spacer is created in the operating room using proprietary molds and low viscosity antibiotic cement to treat patients undergoing a two-stage procedure for infection.
The SpaceFlex Acetabular Cup rounds out the company’s hip offering, with the SpaceFlex Hip already on the market in the U.S. since 2019. As with the Acetabular Cup, SpaceFlex Hip is prepared directly in the operating room to adapt best to the patient’s anatomy. With three mold boxes, it is possible to achieve 36 different configurations.
The SpaceFlex portfolio has been designed to optimize antibiotic elution, and creates a smooth and contoured articulating surface due to the patented mold system. SpaceFlex Knee and Shoulder customizable spacers are also available.
“The approval of the SpaceFlex Acetabular cup is an important step for the US market. It is the first fully disposable and customizable acetabular cement spacer to treat infections. At G-21, we believe there is always room to study, develop, and improve. The SpaceFlex Acetabular Cup is a result of this combined with the drive to provide patients a better quality of life, or as we say, Strength for Life,” commented Filippo Foroni, Co-Founder and Executive Vice President of G-21.
Source: G-21
G-21 received FDA 510(k) clearance to market the SpaceFlex Acetabular Cup. The temporary spacer is created in the operating room using proprietary molds and low viscosity antibiotic cement to treat patients undergoing a two-stage procedure for infection.
The SpaceFlex Acetabular Cup rounds out the company's hip offering, with the SpaceFlex Hip...
G-21 received FDA 510(k) clearance to market the SpaceFlex Acetabular Cup. The temporary spacer is created in the operating room using proprietary molds and low viscosity antibiotic cement to treat patients undergoing a two-stage procedure for infection.
The SpaceFlex Acetabular Cup rounds out the company’s hip offering, with the SpaceFlex Hip already on the market in the U.S. since 2019. As with the Acetabular Cup, SpaceFlex Hip is prepared directly in the operating room to adapt best to the patient’s anatomy. With three mold boxes, it is possible to achieve 36 different configurations.
The SpaceFlex portfolio has been designed to optimize antibiotic elution, and creates a smooth and contoured articulating surface due to the patented mold system. SpaceFlex Knee and Shoulder customizable spacers are also available.
“The approval of the SpaceFlex Acetabular cup is an important step for the US market. It is the first fully disposable and customizable acetabular cement spacer to treat infections. At G-21, we believe there is always room to study, develop, and improve. The SpaceFlex Acetabular Cup is a result of this combined with the drive to provide patients a better quality of life, or as we say, Strength for Life,” commented Filippo Foroni, Co-Founder and Executive Vice President of G-21.
Source: G-21

You are out of free articles for this month
Subscribe as a Guest for $0 and unlock a total of 5 articles per month.
You are out of five articles for this month
Subscribe as an Executive Member for access to unlimited articles, THE ORTHOPAEDIC INDUSTRY ANNUAL REPORT and more.
JV
Julie Vetalice is ORTHOWORLD's Editorial Assistant. She has covered the orthopedic industry for over 20 years, having joined the company in 1999.







