
 Copy to clipboard
Copy to clipboard 
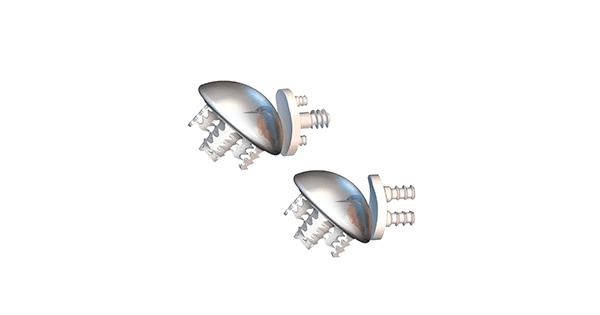
FX received FDA 510(k) clearance to market the Easytech® Stemless Anatomic Shoulder device, the only stemless shoulder on the market with primary peripheral fixation. Easytech has been approved for use in Europe since 2013.
The design of the Easytech Anchor base allows the device to fit peripherally just inside the cortical bone, where bone quality is optimal for fixation.
The cementless Anchor base is available in diameters of 30mm to 42mm. It features a main central post with striaes and five retentive striaes peripherally positioned to help with primary fixation.
The Anchor base can be used with a straight taper connector or a centered spacer, a centered or offset humeral head manufactured from wrought Co-Cr-Mo alloy, TiN coated or uncoated, and a 2-pegs or 3- or 4-pegs cemented glenoid manufactured from ultra-high molecular weight polyethylene for use in an anatomical shoulder configuration.
“This is a very exciting addition to our comprehensive portfolio and will allow us to compete in the stemless market with a tremendous prosthesis that, with its primary peripheral fixation, should give us a competitive advantage and potentially improve patient outcomes with a bone sparing device,” said Baptiste Martin, CEO of FX Shoulder USA.
FX received FDA 510(k) clearance to market the Easytech® Stemless Anatomic Shoulder device, the only stemless shoulder on the market with primary peripheral fixation. Easytech has been approved for use in Europe since 2013.
The design of the Easytech Anchor base allows the device to fit peripherally just inside the cortical bone, where...
FX received FDA 510(k) clearance to market the Easytech® Stemless Anatomic Shoulder device, the only stemless shoulder on the market with primary peripheral fixation. Easytech has been approved for use in Europe since 2013.
The design of the Easytech Anchor base allows the device to fit peripherally just inside the cortical bone, where bone quality is optimal for fixation.
The cementless Anchor base is available in diameters of 30mm to 42mm. It features a main central post with striaes and five retentive striaes peripherally positioned to help with primary fixation.
The Anchor base can be used with a straight taper connector or a centered spacer, a centered or offset humeral head manufactured from wrought Co-Cr-Mo alloy, TiN coated or uncoated, and a 2-pegs or 3- or 4-pegs cemented glenoid manufactured from ultra-high molecular weight polyethylene for use in an anatomical shoulder configuration.
“This is a very exciting addition to our comprehensive portfolio and will allow us to compete in the stemless market with a tremendous prosthesis that, with its primary peripheral fixation, should give us a competitive advantage and potentially improve patient outcomes with a bone sparing device,” said Baptiste Martin, CEO of FX Shoulder USA.

You are out of free articles for this month
Subscribe as a Guest for $0 and unlock a total of 5 articles per month.
You are out of five articles for this month
Subscribe as an Executive Member for access to unlimited articles, THE ORTHOPAEDIC INDUSTRY ANNUAL REPORT and more.
JV
Julie Vetalice is ORTHOWORLD's Editorial Assistant. She has covered the orthopedic industry for over 20 years, having joined the company in 1999.







