
 Copy to clipboard
Copy to clipboard 
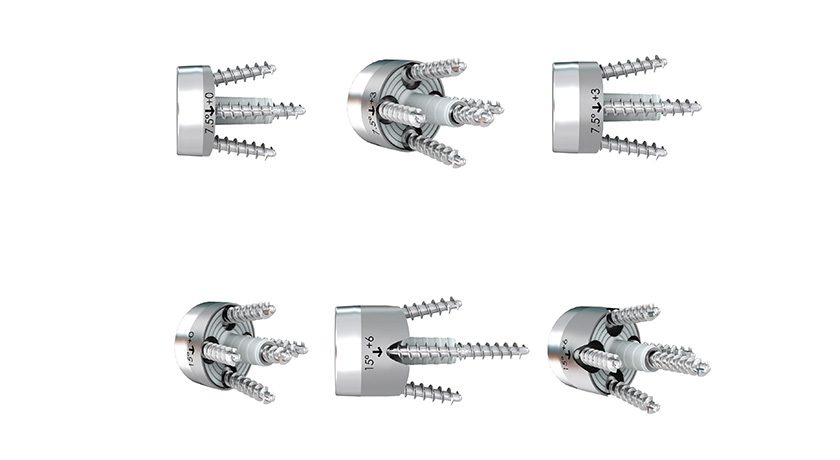
FX received FDA 510(k) clearance clearance to market its full-wedge augmented glenoid baseplates. The newly-cleared baseplates bring six new options to the previously cleared portfolio. There are now a combined total of 18 glenoid baseplate options to the market to address a variety of surgeon needs. Augmented glenoid baseplate options have continued to become a growing solution for surgeons to address bone loss, defects, or complicated morphologies of the glenoid.
The glenoid baseplates are all 24mm in diameter with full-wedge options at 7.5° and 15°, with options to lateralize 0, +3, or +6mm. Each glenoid baseplate has four peripheral screw holes with 12° of polyaxial variability that can be fixed with 4.5mm standard or locking screws. The option to use a 4.5mm central screw can be utilized through the central post for additional fixation (7 central screw length options from 8-20mm in 2mm increments). All glenoid baseplates are made from Ti6AV and have a hydroxyapatite coating.
“This clearance of the full-wedge augmented baseplates further solidifies our portfolio as one of the most comprehensive and innovative shoulder arthroplasty platforms on the market,” said Baptiste Martin, CEO of FX Shoulder Solutions.
Source: FX Shoulder Solutions, Inc.
FX received FDA 510(k) clearance clearance to market its full-wedge augmented glenoid baseplates. The newly-cleared baseplates bring six new options to the previously cleared portfolio. There are now a combined total of 18 glenoid baseplate options to the market to address a variety of surgeon needs. Augmented glenoid baseplate options have...
FX received FDA 510(k) clearance clearance to market its full-wedge augmented glenoid baseplates. The newly-cleared baseplates bring six new options to the previously cleared portfolio. There are now a combined total of 18 glenoid baseplate options to the market to address a variety of surgeon needs. Augmented glenoid baseplate options have continued to become a growing solution for surgeons to address bone loss, defects, or complicated morphologies of the glenoid.
The glenoid baseplates are all 24mm in diameter with full-wedge options at 7.5° and 15°, with options to lateralize 0, +3, or +6mm. Each glenoid baseplate has four peripheral screw holes with 12° of polyaxial variability that can be fixed with 4.5mm standard or locking screws. The option to use a 4.5mm central screw can be utilized through the central post for additional fixation (7 central screw length options from 8-20mm in 2mm increments). All glenoid baseplates are made from Ti6AV and have a hydroxyapatite coating.
“This clearance of the full-wedge augmented baseplates further solidifies our portfolio as one of the most comprehensive and innovative shoulder arthroplasty platforms on the market,” said Baptiste Martin, CEO of FX Shoulder Solutions.
Source: FX Shoulder Solutions, Inc.

You are out of free articles for this month
Subscribe as a Guest for $0 and unlock a total of 5 articles per month.
You are out of five articles for this month
Subscribe as an Executive Member for access to unlimited articles, THE ORTHOPAEDIC INDUSTRY ANNUAL REPORT and more.
JV
Julie Vetalice is ORTHOWORLD's Editorial Assistant. She has covered the orthopedic industry for over 20 years, having joined the company in 1999.







