
 Copy to clipboard
Copy to clipboard 
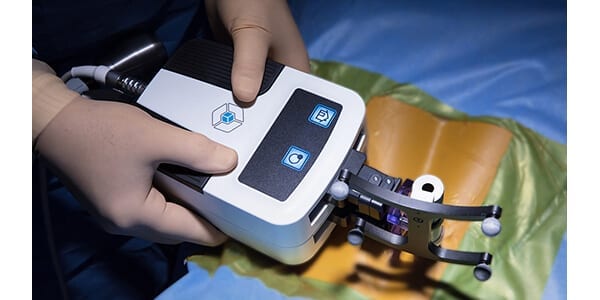
Fusion Robotics was granted FDA 510(k) clearance to market their 3D imaging compatible navigation and robotic targeting system for spine surgery. U.S. launch will occur within the year.
The system addresses key limitations of current spinal navigation and robotics, offering greater procedural efficiency with less expense.
“We appreciate the partnership developed with FDA to rigorously validate safety and accuracy. Next, we look forward to partnering with clinicians and hospitals to increase efficiency, reduce cost and broadly expand the application of robotics to treat patients,” said Brad Clayton, CEO of Fusion Robotics.
Fusion Robotics was granted FDA 510(k) clearance to market their 3D imaging compatible navigation and robotic targeting system for spine surgery. U.S. launch will occur within the year.
The system addresses key limitations of current spinal navigation and robotics, offering greater procedural efficiency with less expense.
"We appreciate...
Fusion Robotics was granted FDA 510(k) clearance to market their 3D imaging compatible navigation and robotic targeting system for spine surgery. U.S. launch will occur within the year.
The system addresses key limitations of current spinal navigation and robotics, offering greater procedural efficiency with less expense.
“We appreciate the partnership developed with FDA to rigorously validate safety and accuracy. Next, we look forward to partnering with clinicians and hospitals to increase efficiency, reduce cost and broadly expand the application of robotics to treat patients,” said Brad Clayton, CEO of Fusion Robotics.

You are out of free articles for this month
Subscribe as a Guest for $0 and unlock a total of 5 articles per month.
You are out of five articles for this month
Subscribe as an Executive Member for access to unlimited articles, THE ORTHOPAEDIC INDUSTRY ANNUAL REPORT and more.
JV
Julie Vetalice is ORTHOWORLD's Editorial Assistant. She has covered the orthopedic industry for over 20 years, having joined the company in 1999.







