Copy to clipboard
Copy to clipboard 
“An average person in the U.S. will experience two fractures in their lifetime.” This was one of the first orthopedic facts that I learned over 20 years ago, and the stat is still quoted online.
Our ability to fall down and smack into things has driven the trauma segment to increased revenue year over year, according to the sales trajectory for trauma as cited in ORTHOWORLD’s Annual Report. Worldwide sales for the segment passed $9 billion in 2024, and are estimated to surpass $10.7 billion in 2028. While the core trauma segment is large and mature, the need for novel solutions remains.
Within the third quarter of this year, the following companies debuted products to help fix our fractures and deformities in scapulae, pelvis and toes throughout our ages.
CurvaFix Low Profile System Gains FDA Clearance
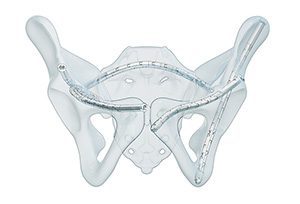 The next-generation CurvaFix Low Profile System is a percutaneous solution for fixation of pelvic fractures, and is built to expand surgical options in challenging scenarios, such as pathological bone, curved or narrow pelvic corridors, intersecting fixation pathways and cases where indwelling or adjacent hardware is present. The new system offers:
The next-generation CurvaFix Low Profile System is a percutaneous solution for fixation of pelvic fractures, and is built to expand surgical options in challenging scenarios, such as pathological bone, curved or narrow pelvic corridors, intersecting fixation pathways and cases where indwelling or adjacent hardware is present. The new system offers:
- 65% smaller head geometry for a lower-profile construct
- Implants with increased compression capability
- Extended implant lengths up to 210mm, enabling connection of intraosseous fixation pathways with a single device
- Patented lock technology with improved visual and tactile confirmation
Clinical experience has shown that the CurvaFix technology provides strong, stable fixation that follows and fills the natural curvature of each patient’s anatomy. This unique approach may reduce pain, support earlier mobility and improve recovery compared to conventional straight implants.
Exactech Granted 510(k) for Scapula Reconstruction
 The Equinoxe Scapula Reconstruction System, which addresses acromial stress fractures, will enter pilot launch with limited availability in the U.S. later this year.
The Equinoxe Scapula Reconstruction System, which addresses acromial stress fractures, will enter pilot launch with limited availability in the U.S. later this year.
The system supports the treatment of scapular fractures using several different techniques, including single and dual plating, regardless of placement of any particular reverse total shoulder arthroplasty (rTSA) implant design. The novel system features a portfolio of anatomically contoured plates in multiple lengths to enable precise fracture management across the range of patient anatomy.
The plate designs incorporate differentiated features to tailor treatment across the Levy Type I, II, IIA and IIB fracture classification patterns. Each contoured plate includes one or more integral hooks (located anteriorly on the acromion, laterally on the acromion and/or a hook along the medial scapular border) to support the scapula and counteract the pull of the deltoid and the biomechanical loading of the rTSA prosthesis. Previous laboratory research has suggested that the use of a lateral hook plate improved fixation of the lateral acromion.
First Case in Clinical Evaluation of HyperFlex Bunion Correction
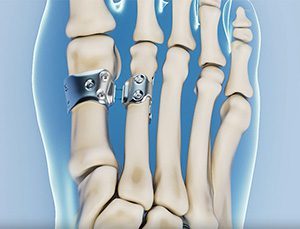 HyperFlex features a flexible, suture-based implant that realigns the metatarsals through a small incision, preserving motion, minimizing trauma, and allowing same-day weight-bearing.
HyperFlex features a flexible, suture-based implant that realigns the metatarsals through a small incision, preserving motion, minimizing trauma, and allowing same-day weight-bearing.
HyperFlex closed a $1.4 million investment in June and received FDA 510(k) marketing clearance in November 2024, the latter under the company name Footbridge Medical. The company holds issued patents in the U.S. and Europe.
J&J MedTech Launches VIRTUGUIDE Patient-Matched Lapidus System in the U.S.
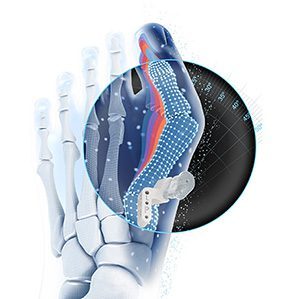 This AI-powered, patient-matched solution uses pre-operative planning software, developed in collaboration with PeekMed, to assess each patient’s bunion and make personalized recommendations for the intended correction.
This AI-powered, patient-matched solution uses pre-operative planning software, developed in collaboration with PeekMed, to assess each patient’s bunion and make personalized recommendations for the intended correction.
Early users of VIRTUGUIDE estimated procedural time savings of at least 30 minutes when using the system compared to their previous technique.
VIRTUGUIDE Software supports a shorter planning window, anatomical visualization, and data-informed decision making. System highlights include:
- AI-automated pre-op planning software in minutes
- Patient matched deformity instrumentation in 24 hours
- Streamlined surgery
The system is the latest addition to JNJ’s portfolio of VELYS Enabling Tech solutions.
OrthoPediatrics Launches 3P Pediatric Plating Platform Hip in the U.S.
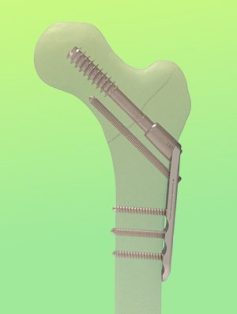 The first surgical case is complete with the new 3P Pediatric Plating Platform Hip System, used to treat proximal femur fractures and deformities.
The first surgical case is complete with the new 3P Pediatric Plating Platform Hip System, used to treat proximal femur fractures and deformities.
3P includes a Beam Screw construct and Locking Proximal Femur Plates available in Infant, Child and Adolescent sizes, and integrates advanced implant and instrument technology to support trauma and deformity surgeries.
Following the system launch, more than 10 cases were booked in August alone, with procedural volume expected to grow substantially in the coming months.
“An average person in the U.S. will experience two fractures in their lifetime.” This was one of the first orthopedic facts that I learned over 20 years ago, and the stat is still quoted online.
Our ability to fall down and smack into things has driven the trauma segment to increased revenue year over year, according to the sales trajectory...
“An average person in the U.S. will experience two fractures in their lifetime.” This was one of the first orthopedic facts that I learned over 20 years ago, and the stat is still quoted online.
Our ability to fall down and smack into things has driven the trauma segment to increased revenue year over year, according to the sales trajectory for trauma as cited in ORTHOWORLD’s Annual Report. Worldwide sales for the segment passed $9 billion in 2024, and are estimated to surpass $10.7 billion in 2028. While the core trauma segment is large and mature, the need for novel solutions remains.
Within the third quarter of this year, the following companies debuted products to help fix our fractures and deformities in scapulae, pelvis and toes throughout our ages.
CurvaFix Low Profile System Gains FDA Clearance
 The next-generation CurvaFix Low Profile System is a percutaneous solution for fixation of pelvic fractures, and is built to expand surgical options in challenging scenarios, such as pathological bone, curved or narrow pelvic corridors, intersecting fixation pathways and cases where indwelling or adjacent hardware is present. The new system offers:
The next-generation CurvaFix Low Profile System is a percutaneous solution for fixation of pelvic fractures, and is built to expand surgical options in challenging scenarios, such as pathological bone, curved or narrow pelvic corridors, intersecting fixation pathways and cases where indwelling or adjacent hardware is present. The new system offers:
- 65% smaller head geometry for a lower-profile construct
- Implants with increased compression capability
- Extended implant lengths up to 210mm, enabling connection of intraosseous fixation pathways with a single device
- Patented lock technology with improved visual and tactile confirmation
Clinical experience has shown that the CurvaFix technology provides strong, stable fixation that follows and fills the natural curvature of each patient’s anatomy. This unique approach may reduce pain, support earlier mobility and improve recovery compared to conventional straight implants.
Exactech Granted 510(k) for Scapula Reconstruction
 The Equinoxe Scapula Reconstruction System, which addresses acromial stress fractures, will enter pilot launch with limited availability in the U.S. later this year.
The Equinoxe Scapula Reconstruction System, which addresses acromial stress fractures, will enter pilot launch with limited availability in the U.S. later this year.
The system supports the treatment of scapular fractures using several different techniques, including single and dual plating, regardless of placement of any particular reverse total shoulder arthroplasty (rTSA) implant design. The novel system features a portfolio of anatomically contoured plates in multiple lengths to enable precise fracture management across the range of patient anatomy.
The plate designs incorporate differentiated features to tailor treatment across the Levy Type I, II, IIA and IIB fracture classification patterns. Each contoured plate includes one or more integral hooks (located anteriorly on the acromion, laterally on the acromion and/or a hook along the medial scapular border) to support the scapula and counteract the pull of the deltoid and the biomechanical loading of the rTSA prosthesis. Previous laboratory research has suggested that the use of a lateral hook plate improved fixation of the lateral acromion.
First Case in Clinical Evaluation of HyperFlex Bunion Correction
 HyperFlex features a flexible, suture-based implant that realigns the metatarsals through a small incision, preserving motion, minimizing trauma, and allowing same-day weight-bearing.
HyperFlex features a flexible, suture-based implant that realigns the metatarsals through a small incision, preserving motion, minimizing trauma, and allowing same-day weight-bearing.
HyperFlex closed a $1.4 million investment in June and received FDA 510(k) marketing clearance in November 2024, the latter under the company name Footbridge Medical. The company holds issued patents in the U.S. and Europe.
J&J MedTech Launches VIRTUGUIDE Patient-Matched Lapidus System in the U.S.
 This AI-powered, patient-matched solution uses pre-operative planning software, developed in collaboration with PeekMed, to assess each patient’s bunion and make personalized recommendations for the intended correction.
This AI-powered, patient-matched solution uses pre-operative planning software, developed in collaboration with PeekMed, to assess each patient’s bunion and make personalized recommendations for the intended correction.
Early users of VIRTUGUIDE estimated procedural time savings of at least 30 minutes when using the system compared to their previous technique.
VIRTUGUIDE Software supports a shorter planning window, anatomical visualization, and data-informed decision making. System highlights include:
- AI-automated pre-op planning software in minutes
- Patient matched deformity instrumentation in 24 hours
- Streamlined surgery
The system is the latest addition to JNJ’s portfolio of VELYS Enabling Tech solutions.
OrthoPediatrics Launches 3P Pediatric Plating Platform Hip in the U.S.
 The first surgical case is complete with the new 3P Pediatric Plating Platform Hip System, used to treat proximal femur fractures and deformities.
The first surgical case is complete with the new 3P Pediatric Plating Platform Hip System, used to treat proximal femur fractures and deformities.
3P includes a Beam Screw construct and Locking Proximal Femur Plates available in Infant, Child and Adolescent sizes, and integrates advanced implant and instrument technology to support trauma and deformity surgeries.
Following the system launch, more than 10 cases were booked in August alone, with procedural volume expected to grow substantially in the coming months.

You’ve reached your limit.
We’re glad you’re finding value in our content — and we’d love for you to keep going.
Subscribe now for unlimited access to orthopedic business intelligence.
JV
Julie Vetalice is ORTHOWORLD's Editorial Assistant. She has covered the orthopedic industry for over 20 years, having joined the company in 1999.