
 Copy to clipboard
Copy to clipboard 
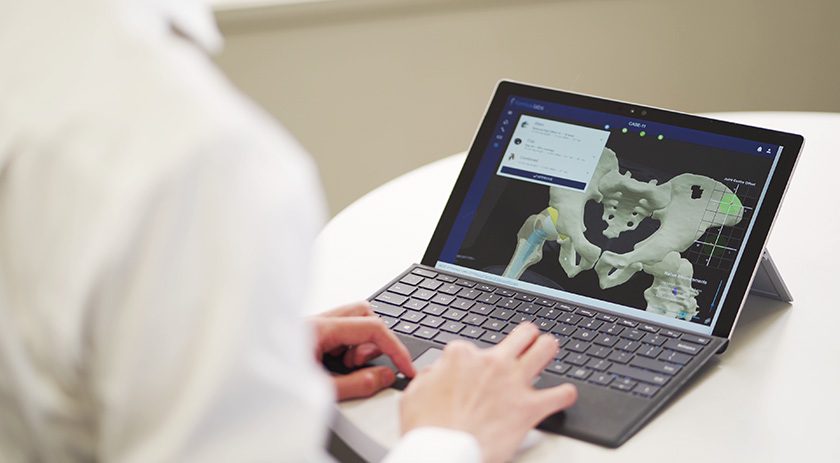
Formus Labs received FDA 510(k) clearance to market Formus Hip, described as the first “automated radiological image processing software” for hip replacement pre-op planning.
Formus is designed to expedite the joint replacement planning process by combining artificial intelligence and computational biomechanics to calculate a patient’s implant fit and deliver digestible, actionable and fully-interactive 3D surgical plans. This pre-operative planning technology provides a surgeon with a personalized curated patient surgical plan before the patient enters the operating room. This technology provides accurate outputs from scan to plan in under an hour.
“Today is a huge milestone in our journey to bring cutting-edge, pre-op surgery planning tools to surgeons, not only to make their work easier and more efficient, but also has the potential to improve the outcomes for their patients,” said Dr. Ju Zhang, founder and CEO of Formus Labs. “FDA clearance serves as significant validation of the accuracy and rigor of our AI models. The surgeons who have used the Formus platform in Australia and New Zealand tell us they like having pre-op plans that make facing any unforeseen challenges on the day of the surgery easier to overcome because of the thorough understanding of each patient’s physiology. It also has huge potential to save costs, time spent on logistics, and inventory. We’re excited to bring those same potential savings to providers in the US now too.”
“The technology underlying the Formus solution helps drive potential improvement in orthopedic patient outcomes. We’re thrilled the 510(k) approval now allows us to fully unlock our US commercialization strategy to achieve positive outcomes for more patients,” Vignesh Kumar, co-managing partner at GD1 and Formus board member. ”Formus is a great example of a digital health solution that balances the delicate ‘iron triangle’ of cost, access, and quality while addressing those core pain points in an elegant manner.”
Source: Formus Labs
Formus Labs received FDA 510(k) clearance to market Formus Hip, described as the first “automated radiological image processing software” for hip replacement pre-op planning.
Formus is designed to expedite the joint replacement planning process by combining artificial intelligence and computational biomechanics to calculate a patient’s implant...
Formus Labs received FDA 510(k) clearance to market Formus Hip, described as the first “automated radiological image processing software” for hip replacement pre-op planning.
Formus is designed to expedite the joint replacement planning process by combining artificial intelligence and computational biomechanics to calculate a patient’s implant fit and deliver digestible, actionable and fully-interactive 3D surgical plans. This pre-operative planning technology provides a surgeon with a personalized curated patient surgical plan before the patient enters the operating room. This technology provides accurate outputs from scan to plan in under an hour.
“Today is a huge milestone in our journey to bring cutting-edge, pre-op surgery planning tools to surgeons, not only to make their work easier and more efficient, but also has the potential to improve the outcomes for their patients,” said Dr. Ju Zhang, founder and CEO of Formus Labs. “FDA clearance serves as significant validation of the accuracy and rigor of our AI models. The surgeons who have used the Formus platform in Australia and New Zealand tell us they like having pre-op plans that make facing any unforeseen challenges on the day of the surgery easier to overcome because of the thorough understanding of each patient’s physiology. It also has huge potential to save costs, time spent on logistics, and inventory. We’re excited to bring those same potential savings to providers in the US now too.”
“The technology underlying the Formus solution helps drive potential improvement in orthopedic patient outcomes. We’re thrilled the 510(k) approval now allows us to fully unlock our US commercialization strategy to achieve positive outcomes for more patients,” Vignesh Kumar, co-managing partner at GD1 and Formus board member. ”Formus is a great example of a digital health solution that balances the delicate ‘iron triangle’ of cost, access, and quality while addressing those core pain points in an elegant manner.”
Source: Formus Labs

You are out of free articles for this month
Subscribe as a Guest for $0 and unlock a total of 5 articles per month.
You are out of five articles for this month
Subscribe as an Executive Member for access to unlimited articles, THE ORTHOPAEDIC INDUSTRY ANNUAL REPORT and more.
JV
Julie Vetalice is ORTHOWORLD's Editorial Assistant. She has covered the orthopedic industry for over 20 years, having joined the company in 1999.







