
 Copy to clipboard
Copy to clipboard 
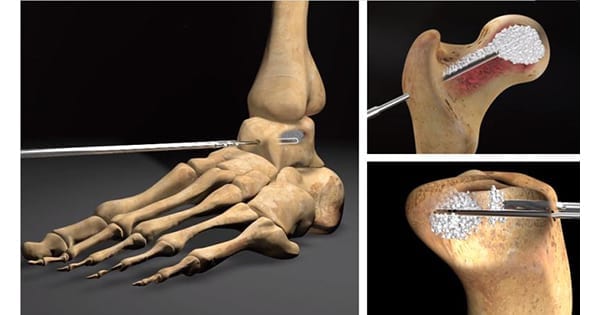
Flow-FX introduced FLOW-DRILL™, a low-trauma option for precision decompression and biologic delivery.
The patented FLOW-DRILL is a Class 1, FDA 510(k) exempt device designed for use with any HMT/Zimmer-Hall handle. It supports delivery of biologics into bone damaged by avascular necrosis, subchondral lesions and other bone conditions. It may also be used for precision biopsies, bone marrow aspiration and aspiration of bone cysts.
FLOW-DRILL is available in diameters 3.6mm and 4.8mm, with 2.4mm and 3.0mm to come. Each kit contains three Guide wires, and is single use/gamma sterilized for convenience.
The system allows for successful treatment while minimizing risk of collapse and fracture, and is designed to facilitate precise, low-impact delivery of healing biologic materials and bone void filler. A patented Luer fitting enables direct delivery using a standard syringe.
The company’s other products for biologic/bone void filler delivery include the 2-CAN™ multi-channeled cannula, FLOW-SCREW™ and FLOW-NAIL™ systems and FLOW-FX2™ calcium phosphate bone void filler.
Flow-FX introduced FLOW-DRILL™, a low-trauma option for precision decompression and biologic delivery.
The patented FLOW-DRILL is a Class 1, FDA 510(k) exempt device designed for use with any HMT/Zimmer-Hall handle. It supports delivery of biologics into bone damaged by avascular necrosis, subchondral lesions and other bone conditions. It may...
Flow-FX introduced FLOW-DRILL™, a low-trauma option for precision decompression and biologic delivery.
The patented FLOW-DRILL is a Class 1, FDA 510(k) exempt device designed for use with any HMT/Zimmer-Hall handle. It supports delivery of biologics into bone damaged by avascular necrosis, subchondral lesions and other bone conditions. It may also be used for precision biopsies, bone marrow aspiration and aspiration of bone cysts.
FLOW-DRILL is available in diameters 3.6mm and 4.8mm, with 2.4mm and 3.0mm to come. Each kit contains three Guide wires, and is single use/gamma sterilized for convenience.
The system allows for successful treatment while minimizing risk of collapse and fracture, and is designed to facilitate precise, low-impact delivery of healing biologic materials and bone void filler. A patented Luer fitting enables direct delivery using a standard syringe.
The company’s other products for biologic/bone void filler delivery include the 2-CAN™ multi-channeled cannula, FLOW-SCREW™ and FLOW-NAIL™ systems and FLOW-FX2™ calcium phosphate bone void filler.

You are out of free articles for this month
Subscribe as a Guest for $0 and unlock a total of 5 articles per month.
You are out of five articles for this month
Subscribe as an Executive Member for access to unlimited articles, THE ORTHOPAEDIC INDUSTRY ANNUAL REPORT and more.
JV
Julie Vetalice is ORTHOWORLD's Editorial Assistant. She has covered the orthopedic industry for over 20 years, having joined the company in 1999.







