
 Copy to clipboard
Copy to clipboard 
Flow-FX was issued a new FDA 510(k) clearance, K221115. The patent-pending Flow-Nail™ with LAGLOCK™ is reportedly already the only trochanteric nail system patented and 510(k)-cleared for fracture fixation and delivery of bone void fillers. In addition, it now has what Flow-FX claims is the first and only through-the-lag screw static locking mechanism.
The 510(k) related testing showed that this enhancement maintains Flow-Nail’s fatigue and static strength characteristics while providing previously unachievable axial strength. Comparing LAGLOCK to other static mechanisms on the market that tend to slip, or create stress points for screw breakage, and fail at a much lower load, the LAGLOCK did not slip or fail, but did max out an axial load cell at 440lb.
Many studies have shown reduced screw cutout with femoral head augmentation, while others have shown the value of a static locking mechanism for certain unstable fractures. Flow-Nail with LAGLOCK now combines both technologies.
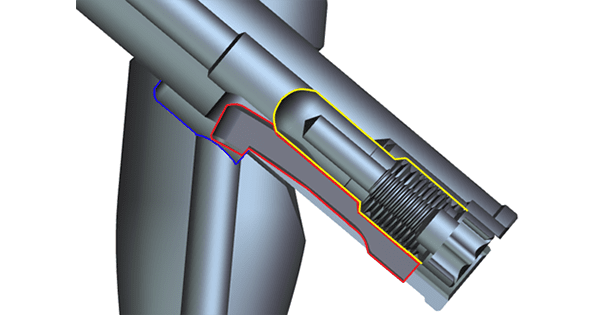
In the image above, the Lag Screw Hook (depicted in red) is deployed with Peg (depicted in yellow) into pocket of Trochanteric IM Nail (depicted in blue).
Flow-FX offers a suite of instruments and implants designed for precision delivery to bone, including Flow-Nail, Flow-Screw™, Flow-Drill™ and 2-CAN™.
Source: FlowFX LLC
Flow-FX was issued a new FDA 510(k) clearance, K221115. The patent-pending Flow-Nail™ with LAGLOCK™ is reportedly already the only trochanteric nail system patented and 510(k)-cleared for fracture fixation and delivery of bone void fillers. In addition, it now has what Flow-FX claims is the first and only through-the-lag screw static locking...
Flow-FX was issued a new FDA 510(k) clearance, K221115. The patent-pending Flow-Nail™ with LAGLOCK™ is reportedly already the only trochanteric nail system patented and 510(k)-cleared for fracture fixation and delivery of bone void fillers. In addition, it now has what Flow-FX claims is the first and only through-the-lag screw static locking mechanism.
The 510(k) related testing showed that this enhancement maintains Flow-Nail’s fatigue and static strength characteristics while providing previously unachievable axial strength. Comparing LAGLOCK to other static mechanisms on the market that tend to slip, or create stress points for screw breakage, and fail at a much lower load, the LAGLOCK did not slip or fail, but did max out an axial load cell at 440lb.
Many studies have shown reduced screw cutout with femoral head augmentation, while others have shown the value of a static locking mechanism for certain unstable fractures. Flow-Nail with LAGLOCK now combines both technologies.
In the image above, the Lag Screw Hook (depicted in red) is deployed with Peg (depicted in yellow) into pocket of Trochanteric IM Nail (depicted in blue).
Flow-FX offers a suite of instruments and implants designed for precision delivery to bone, including Flow-Nail, Flow-Screw™, Flow-Drill™ and 2-CAN™.
Source: FlowFX LLC

You are out of free articles for this month
Subscribe as a Guest for $0 and unlock a total of 5 articles per month.
You are out of five articles for this month
Subscribe as an Executive Member for access to unlimited articles, THE ORTHOPAEDIC INDUSTRY ANNUAL REPORT and more.
JV
Julie Vetalice is ORTHOWORLD's Editorial Assistant. She has covered the orthopedic industry for over 20 years, having joined the company in 1999.







