
 Copy to clipboard
Copy to clipboard 
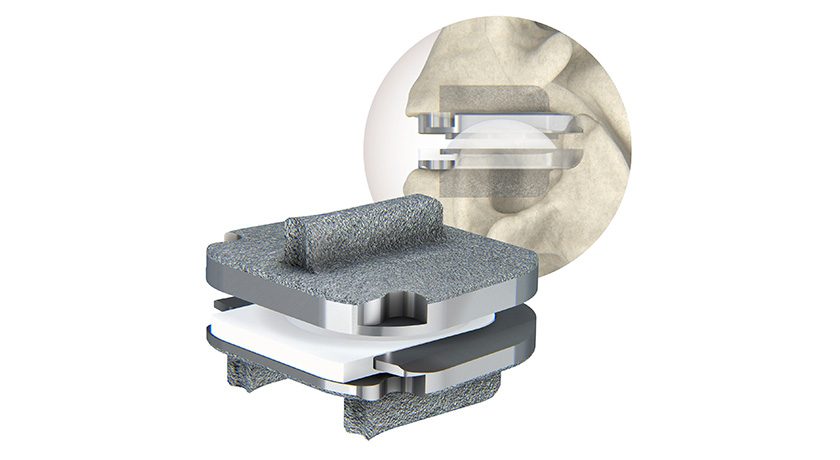
Centinel Spine announced the first U.S. implantation of its prodisc C Nova cervical total disc replacement (TDR). In 2022, the company received FDA approval for 1-level indications for the prodisc C Nova device.
The prodisc C Nova technology is Centinel Spine’s fourth cervical TDR offering, and the third new cervical TDR product released in the U.S. since late 2022. The prodisc C Vivo and prodisc C SK products were both made available on a limited basis in late 2022 and have been used in over 8,000 procedures since release.
Similar to all prodisc products, the prodisc C Nova device incorporates prodisc CORE technology, the basis behind the predictable clinical outcomes of the prodisc platform after 30 years and over 250,000 implantations worldwide.
Centinel Spine CEO Steve Murray said, “We are delighted to make another proven prodisc cervical option available in the U.S. Our experience with prodisc C Vivo and prodisc C SK has shown that surgeons appreciate the variety of endplate designs for their consideration and selection in treating each patient. And, importantly, the entire prodisc portfolio is built on the proven, safe, and reliable motion preservation design that makes prodisc such a trusted name in spine care.”
Source: Centinel Spine, LLC
Centinel Spine announced the first U.S. implantation of its prodisc C Nova cervical total disc replacement (TDR). In 2022, the company received FDA approval for 1-level indications for the prodisc C Nova device.
The prodisc C Nova technology is Centinel Spine's fourth cervical TDR offering, and the third new cervical TDR product released...
Centinel Spine announced the first U.S. implantation of its prodisc C Nova cervical total disc replacement (TDR). In 2022, the company received FDA approval for 1-level indications for the prodisc C Nova device.
The prodisc C Nova technology is Centinel Spine’s fourth cervical TDR offering, and the third new cervical TDR product released in the U.S. since late 2022. The prodisc C Vivo and prodisc C SK products were both made available on a limited basis in late 2022 and have been used in over 8,000 procedures since release.
Similar to all prodisc products, the prodisc C Nova device incorporates prodisc CORE technology, the basis behind the predictable clinical outcomes of the prodisc platform after 30 years and over 250,000 implantations worldwide.
Centinel Spine CEO Steve Murray said, “We are delighted to make another proven prodisc cervical option available in the U.S. Our experience with prodisc C Vivo and prodisc C SK has shown that surgeons appreciate the variety of endplate designs for their consideration and selection in treating each patient. And, importantly, the entire prodisc portfolio is built on the proven, safe, and reliable motion preservation design that makes prodisc such a trusted name in spine care.”
Source: Centinel Spine, LLC

You are out of free articles for this month
Subscribe as a Guest for $0 and unlock a total of 5 articles per month.
You are out of five articles for this month
Subscribe as an Executive Member for access to unlimited articles, THE ORTHOPAEDIC INDUSTRY ANNUAL REPORT and more.
JV
Julie Vetalice is ORTHOWORLD's Editorial Assistant. She has covered the orthopedic industry for over 20 years, having joined the company in 1999.







