
 Copy to clipboard
Copy to clipboard 
The first U.S. patient has received the BioPoly Partial Resurfacing Knee Implant as part of an FDA-approved randomized controlled trial being conducted at multiple sites across the country.
Although the BioPoly Partial Resurfacing Knee Implant is new to the U.S., it was first implanted in Europe in 2012 and was part of a registry study. Positive long-term (5 years) clinical results from this study have been published in The Journal of Bone and Joint Surgery, a prestigious orthopedic journal. From the published European results, BioPoly knee patients were able to immediately bear weight and had no restrictions in their lifestyle. They saw significant improvements in quality of life and reduction in pain, and these positive outcomes were maintained or even continued to improve out to 5 years.
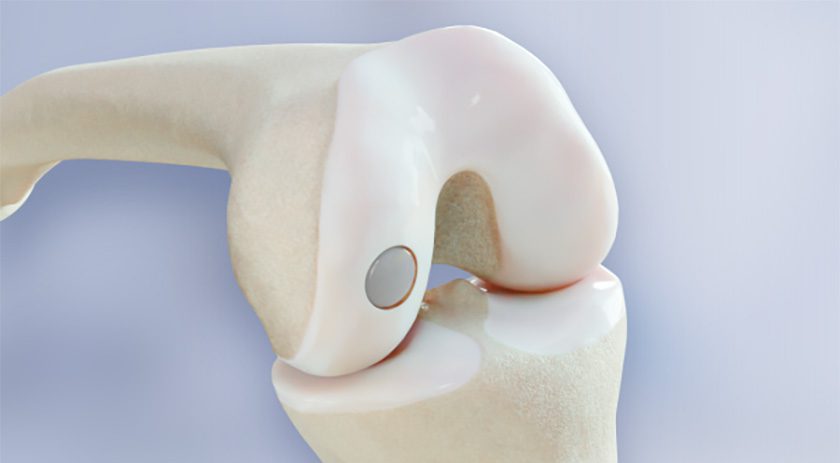
BioPoly knee implants are manufactured from a proprietary synthetic cartilage material and are designed to repair areas in the knee where the cartilage is damaged and causes debilitating pain. So instead of replacing the entire joint, BioPoly just replaces the damaged portion, thus preserving the patient’s natural joint tissue.
According to Dr. Herb Schwartz, BioPoly President & CEO, “This is an extremely important milestone for the Company because upon FDA approval, BioPoly will be positioned to introduce its synthetic cartilage knee implant in the United States, offering patients a motion-preserving alternative to total or partial knee replacements.” The initiation of this study marks the beginning of what could be a paradigm shift in joint preservation as leading orthopedic surgeons across the U.S. are now evaluating BioPoly’s unique material technology in patients who would otherwise face limited treatment options.
BioPoly’s COO, Sheila Schwartz, said, “We are thrilled that the very first U.S. patient to receive our BioPoly Partial Resurfacing Knee Implant was in Fort Wayne,” where BioPoly is headquartered. With a planned enrollment of 152 patients, this study represents the pivotal step toward FDA approval and U.S. commercialization of BioPoly’s novel knee implant.
Source: BioPoly LLC
The first U.S. patient has received the BioPoly Partial Resurfacing Knee Implant as part of an FDA-approved randomized controlled trial being conducted at multiple sites across the country.
Although the BioPoly Partial Resurfacing Knee Implant is new to the U.S., it was first implanted in Europe in 2012 and was part of a registry study....
The first U.S. patient has received the BioPoly Partial Resurfacing Knee Implant as part of an FDA-approved randomized controlled trial being conducted at multiple sites across the country.
Although the BioPoly Partial Resurfacing Knee Implant is new to the U.S., it was first implanted in Europe in 2012 and was part of a registry study. Positive long-term (5 years) clinical results from this study have been published in The Journal of Bone and Joint Surgery, a prestigious orthopedic journal. From the published European results, BioPoly knee patients were able to immediately bear weight and had no restrictions in their lifestyle. They saw significant improvements in quality of life and reduction in pain, and these positive outcomes were maintained or even continued to improve out to 5 years.
BioPoly knee implants are manufactured from a proprietary synthetic cartilage material and are designed to repair areas in the knee where the cartilage is damaged and causes debilitating pain. So instead of replacing the entire joint, BioPoly just replaces the damaged portion, thus preserving the patient’s natural joint tissue.
According to Dr. Herb Schwartz, BioPoly President & CEO, “This is an extremely important milestone for the Company because upon FDA approval, BioPoly will be positioned to introduce its synthetic cartilage knee implant in the United States, offering patients a motion-preserving alternative to total or partial knee replacements.” The initiation of this study marks the beginning of what could be a paradigm shift in joint preservation as leading orthopedic surgeons across the U.S. are now evaluating BioPoly’s unique material technology in patients who would otherwise face limited treatment options.
BioPoly’s COO, Sheila Schwartz, said, “We are thrilled that the very first U.S. patient to receive our BioPoly Partial Resurfacing Knee Implant was in Fort Wayne,” where BioPoly is headquartered. With a planned enrollment of 152 patients, this study represents the pivotal step toward FDA approval and U.S. commercialization of BioPoly’s novel knee implant.
Source: BioPoly LLC

You’ve reached your limit.
We’re glad you’re finding value in our content — and we’d love for you to keep going.
Subscribe now for unlimited access to orthopedic business intelligence.
JV
Julie Vetalice is ORTHOWORLD's Editorial Assistant. She has covered the orthopedic industry for over 20 years, having joined the company in 1999.







