
 Copy to clipboard
Copy to clipboard 
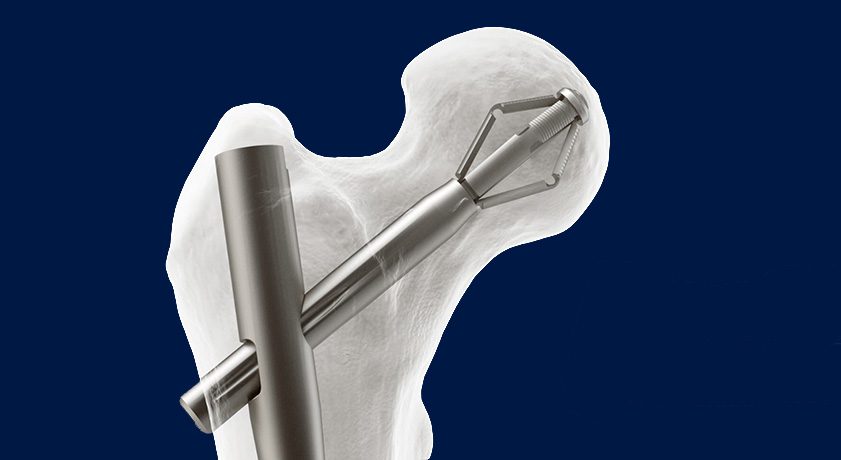
X-Bolt Orthopedics announced the successful completion of the first US implant using its Pro-X1 Trochanteric Nailing System. This milestone marks the official commercial launch of the company’s flagship product.
The Pro-X1 system offers stronger anchorage and rotational stability in osteoporotic hip fracture fixation compared to traditional screw-based methods. This is especially beneficial for frail, elderly patients, as the expanding bolt provides superior fixation, addressing one of the major challenges in hip fracture treatment.
Pro-X1 builds on the company’s expanding bolt design, which was clinically proven in the largest-ever hip fracture randomized controlled trial, with the lowest-ever cut-out rate published for hip fractures at 0.8%.
Commenting on the milestone, Dr. Brian Thornes, X-Bolt’s founder and previous inventor of the globally recognized TightRope, said: “Orthopedic trauma has seen the treatment of ankle injuries revolutionized with the TightRope suture-button, and should expect to see a similar paradigm shift now in the treatment of hip fractures with our expanding bolt and the Pro-X1 system. Hip fractures affect over 200,000 patients annually in the U.S., and this will bring a much-needed advancement in patient care.”
The Pro-X1 Trochanteric Nailing System received FDA 510(k) clearance in 2023, and the completion of this first US surgery marks the beginning of a limited commercial release. X-Bolt plans to scale its operations in the coming months, deploying additional inventory and expanding its reach to more markets across the United States.
Source: X-Bolt
X-Bolt Orthopedics announced the successful completion of the first US implant using its Pro-X1 Trochanteric Nailing System. This milestone marks the official commercial launch of the company's flagship product.
The Pro-X1 system offers stronger anchorage and rotational stability in osteoporotic hip fracture fixation compared to traditional...
X-Bolt Orthopedics announced the successful completion of the first US implant using its Pro-X1 Trochanteric Nailing System. This milestone marks the official commercial launch of the company’s flagship product.
The Pro-X1 system offers stronger anchorage and rotational stability in osteoporotic hip fracture fixation compared to traditional screw-based methods. This is especially beneficial for frail, elderly patients, as the expanding bolt provides superior fixation, addressing one of the major challenges in hip fracture treatment.
Pro-X1 builds on the company’s expanding bolt design, which was clinically proven in the largest-ever hip fracture randomized controlled trial, with the lowest-ever cut-out rate published for hip fractures at 0.8%.
Commenting on the milestone, Dr. Brian Thornes, X-Bolt’s founder and previous inventor of the globally recognized TightRope, said: “Orthopedic trauma has seen the treatment of ankle injuries revolutionized with the TightRope suture-button, and should expect to see a similar paradigm shift now in the treatment of hip fractures with our expanding bolt and the Pro-X1 system. Hip fractures affect over 200,000 patients annually in the U.S., and this will bring a much-needed advancement in patient care.”
The Pro-X1 Trochanteric Nailing System received FDA 510(k) clearance in 2023, and the completion of this first US surgery marks the beginning of a limited commercial release. X-Bolt plans to scale its operations in the coming months, deploying additional inventory and expanding its reach to more markets across the United States.
Source: X-Bolt

You are out of free articles for this month
Subscribe as a Guest for $0 and unlock a total of 5 articles per month.
You are out of five articles for this month
Subscribe as an Executive Member for access to unlimited articles, THE ORTHOPAEDIC INDUSTRY ANNUAL REPORT and more.
JV
Julie Vetalice is ORTHOWORLD's Editorial Assistant. She has covered the orthopedic industry for over 20 years, having joined the company in 1999.







