Copy to clipboard
Copy to clipboard 
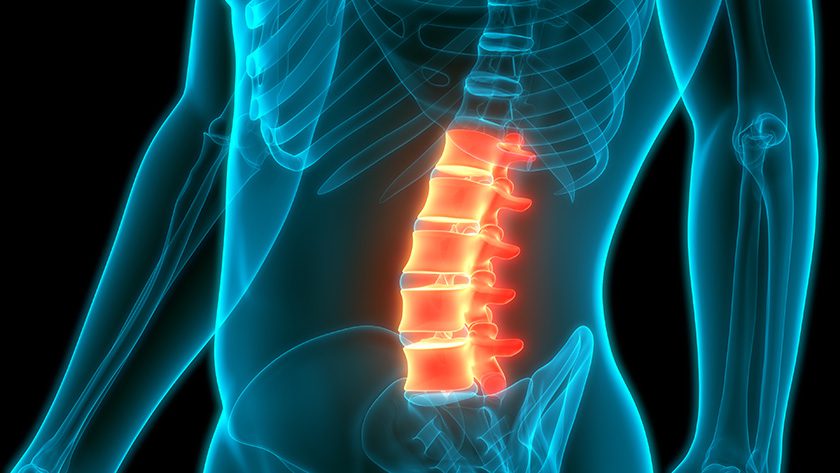
Cerapedics announced the first patient treated with PearlMatrix P-15 Peptide Enhanced Bone Graft following its recent FDA approval. PearlMatrix Bone Graft is a Class III drug/device combination product for lumbar fusion.
Of the more than 350 spinal bone grafts in the U.S. market, Cerapedics has two of only three spinal bone grafts that have successfully gone through the PMA process.
“This first surgery post-approval is a significant milestone for Cerapedics,” said Valeska Schroeder, Chief Executive Officer of Cerapedics. “Every single team member behind the development, approval, and distribution of PearlMatrix P-15 Peptide Enhanced Bone Graft worked tirelessly to get this unique drug-device into the hands of surgeons to help them safely and effectively treat their patients. We are proud of the innovation we bring to the market, and we look forward to seeing the positive impact of our devices.”
Source: Cerapedics
Cerapedics announced the first patient treated with PearlMatrix P-15 Peptide Enhanced Bone Graft following its recent FDA approval. PearlMatrix Bone Graft is a Class III drug/device combination product for lumbar fusion.
Of the more than 350 spinal bone grafts in the U.S. market, Cerapedics has two of only three spinal bone grafts that...
Cerapedics announced the first patient treated with PearlMatrix P-15 Peptide Enhanced Bone Graft following its recent FDA approval. PearlMatrix Bone Graft is a Class III drug/device combination product for lumbar fusion.
Of the more than 350 spinal bone grafts in the U.S. market, Cerapedics has two of only three spinal bone grafts that have successfully gone through the PMA process.
“This first surgery post-approval is a significant milestone for Cerapedics,” said Valeska Schroeder, Chief Executive Officer of Cerapedics. “Every single team member behind the development, approval, and distribution of PearlMatrix P-15 Peptide Enhanced Bone Graft worked tirelessly to get this unique drug-device into the hands of surgeons to help them safely and effectively treat their patients. We are proud of the innovation we bring to the market, and we look forward to seeing the positive impact of our devices.”
Source: Cerapedics

You are out of free articles for this month
Subscribe as a Guest for $0 and unlock a total of 5 articles per month.
You are out of five articles for this month
Subscribe as an Executive Member for access to unlimited articles, THE ORTHOPAEDIC INDUSTRY ANNUAL REPORT and more.
JV
Julie Vetalice is ORTHOWORLD's Editorial Assistant. She has covered the orthopedic industry for over 20 years, having joined the company in 1999.