
 Copy to clipboard
Copy to clipboard 
The ZSFab Cervical Interbody System has been successfully used in its first U.S. clinical case. The system received FDA 510(k) market clearance in early 2021. Prior to this first U.S. clinical use, the ZSFab Cervical Interbody System had already been successfully implanted in clinical cases in China.
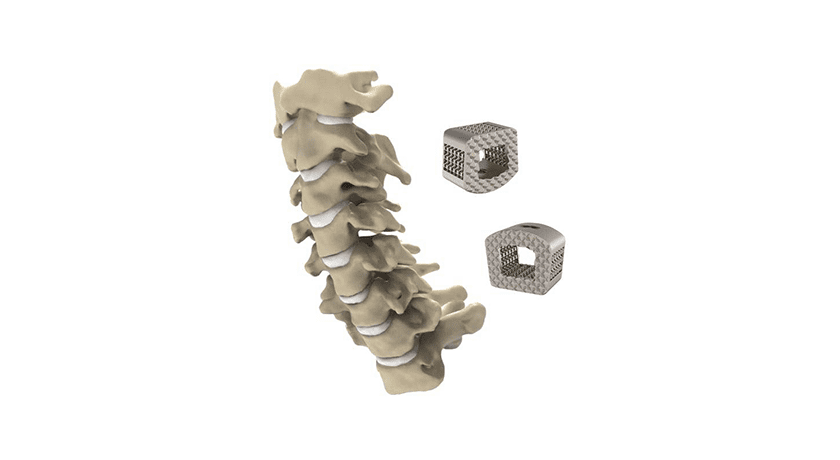
The ZSFab Cervical Interbody System features an additively manufactured porous titanium implant with an optimized triply periodic minimal surface (TPMS) lattice structure, reducing subsidence and improving stress distribution. Additionally, the ZSFab cervical implant has rigid teeth on the inferior and superior surfaces to resist migration after insertion as well as to maximize contact surface area with the vertebral endplates. The interbody device is available in a multitude of sizes for anterior cervical discectomy and fusion.
Jing Zhang, PhD, CEO of ZSFab, said, “The device is designed with interconnected porous structures, engineered for bony integration, minimal debris, high fatigue strength, and minimized subsidence. As we expand our spine solutions, our team is dedicated to providing products of the highest quality to improve patient outcomes with shortened recovery time and reduced revision rates.”
Source: ZSFab
The ZSFab Cervical Interbody System has been successfully used in its first U.S. clinical case. The system received FDA 510(k) market clearance in early 2021. Prior to this first U.S. clinical use, the ZSFab Cervical Interbody System had already been successfully implanted in clinical cases in China.
The ZSFab Cervical Interbody System...
The ZSFab Cervical Interbody System has been successfully used in its first U.S. clinical case. The system received FDA 510(k) market clearance in early 2021. Prior to this first U.S. clinical use, the ZSFab Cervical Interbody System had already been successfully implanted in clinical cases in China.
The ZSFab Cervical Interbody System features an additively manufactured porous titanium implant with an optimized triply periodic minimal surface (TPMS) lattice structure, reducing subsidence and improving stress distribution. Additionally, the ZSFab cervical implant has rigid teeth on the inferior and superior surfaces to resist migration after insertion as well as to maximize contact surface area with the vertebral endplates. The interbody device is available in a multitude of sizes for anterior cervical discectomy and fusion.
Jing Zhang, PhD, CEO of ZSFab, said, “The device is designed with interconnected porous structures, engineered for bony integration, minimal debris, high fatigue strength, and minimized subsidence. As we expand our spine solutions, our team is dedicated to providing products of the highest quality to improve patient outcomes with shortened recovery time and reduced revision rates.”
Source: ZSFab

You are out of free articles for this month
Subscribe as a Guest for $0 and unlock a total of 5 articles per month.
You are out of five articles for this month
Subscribe as an Executive Member for access to unlimited articles, THE ORTHOPAEDIC INDUSTRY ANNUAL REPORT and more.
JV
Julie Vetalice is ORTHOWORLD's Editorial Assistant. She has covered the orthopedic industry for over 20 years, having joined the company in 1999.







