Copy to clipboard
Copy to clipboard 
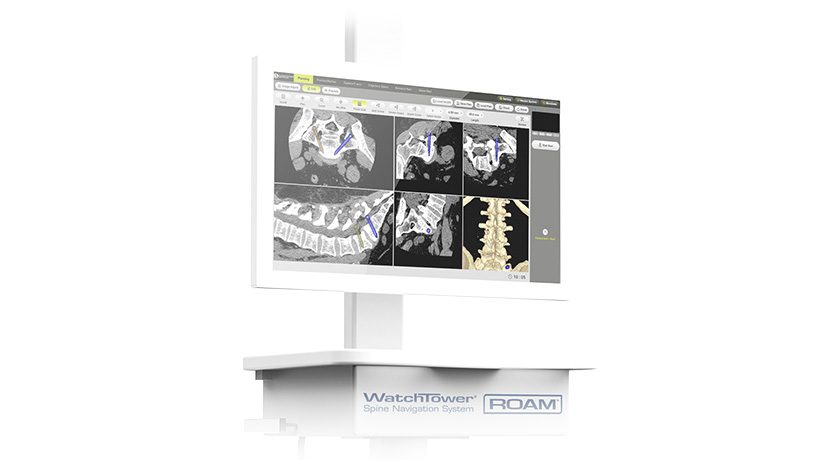
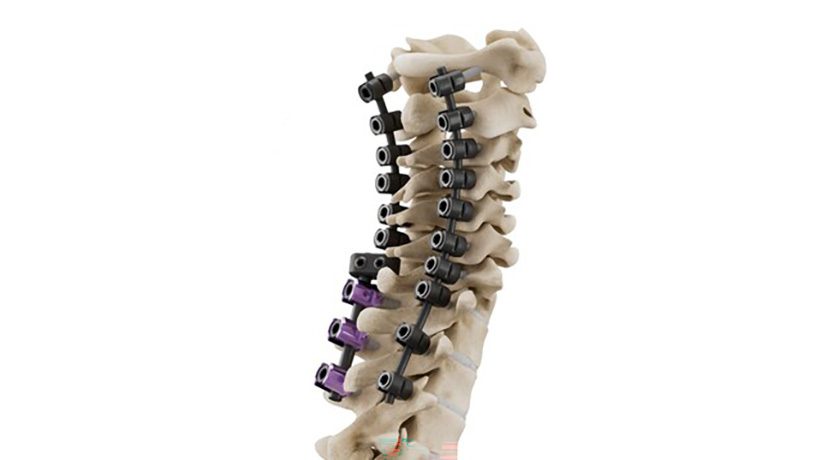
First Ray received FDA 510(k) clearance for the CortiClamp™ System for small bone fixation.
CortiClamp is reportedly the first bone screw system to include a second “screw head” that is attached to the distal end of the screw, providing heads on both ends of the shaft to enhance bi-cortical clamping force. To reduce procedural steps, the system includes an all-in-one instrument comprising reduction clamp, drill guide, countersink guide, depth gage and implant delivery guide.
First Ray is a development stage device company incubated and operated by Surgical Frontiers, whose portfolio also includes KATOR.
Sources: First Ray, ORTHOWORLD
First Ray received FDA 510(k) clearance for the CortiClamp™ System for small bone fixation.
CortiClamp is reportedly the first bone screw system to include a second "screw head" that is attached to the distal end of the screw, providing heads on both ends of the shaft to enhance bi-cortical clamping force. To reduce procedural steps, the...
First Ray received FDA 510(k) clearance for the CortiClamp™ System for small bone fixation.
CortiClamp is reportedly the first bone screw system to include a second “screw head” that is attached to the distal end of the screw, providing heads on both ends of the shaft to enhance bi-cortical clamping force. To reduce procedural steps, the system includes an all-in-one instrument comprising reduction clamp, drill guide, countersink guide, depth gage and implant delivery guide.
First Ray is a development stage device company incubated and operated by Surgical Frontiers, whose portfolio also includes KATOR.
Sources: First Ray, ORTHOWORLD

You’ve reached your limit.
We’re glad you’re finding value in our content — and we’d love for you to keep going.
Subscribe now for unlimited access to orthopedic business intelligence.
JV
Julie Vetalice is ORTHOWORLD's Editorial Assistant. She has covered the orthopedic industry for over 20 years, having joined the company in 1999.