Copy to clipboard
Copy to clipboard 
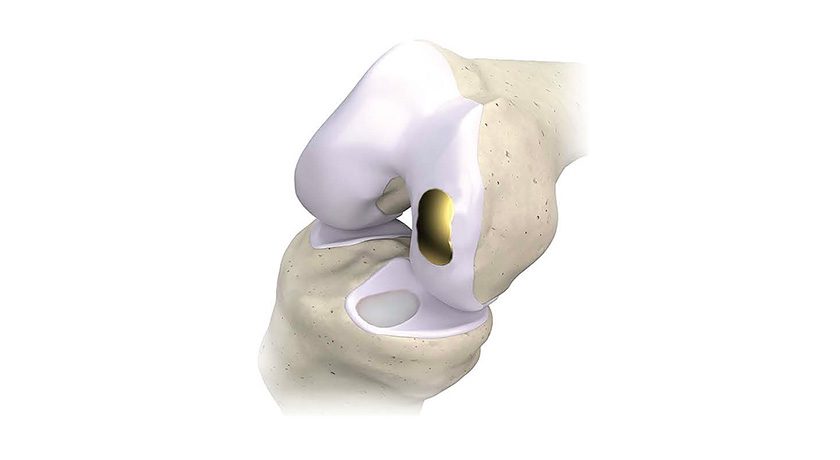
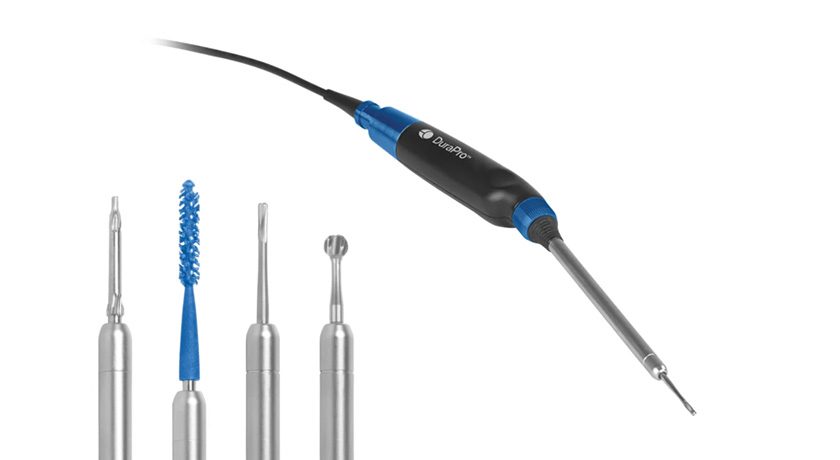
Integra LifeSciences announced initial surgeries performed with the Integra® XT Revision Total Ankle Replacement system.
Integra XT represents one of the first FDA-cleared devices indicated specifically for revision arthroplasty, and can be used to revise any commercially available primary total ankle system. Features include an augmented posterior sloped talus to address subsidence by rebuilding posterior talar height, and an anatomic design that mimics natural ankle kinematics. The system received FDA 510(k) clearance in 2016 as the Salto XT; Integra had acquired U.S. rights to the system from Tornier in 2015.
For the nine months ended September 30, 2018, Integra posted Joint Recon Extremities revenue of $27.6MM, up 2.4% from the prior year, and Trauma device revenue of $43.7MM, -5.8%. The company lowered their overall organic revenue growth goal for the year, in part due to disappointing performance in their orthopaedic business. Within the segment, growth in U.S. ankle and shoulder product lines was not enough to offset a mid-teens decline in the lower extremity fixation portfolio. Leadership expects improved performance in 2019, aided by new products like Integra XT Revision and the Panta II fusion nail.
Sources: Integra LifeSciences Corp.; ORTHOWORLD Inc.
Integra LifeSciences announced initial surgeries performed with the Integra® XT Revision Total Ankle Replacement system.
Integra XT represents one of the first FDA-cleared devices indicated specifically for revision arthroplasty, and can be used to revise any commercially available primary total ankle system. Features include an augmented...
Integra LifeSciences announced initial surgeries performed with the Integra® XT Revision Total Ankle Replacement system.
Integra XT represents one of the first FDA-cleared devices indicated specifically for revision arthroplasty, and can be used to revise any commercially available primary total ankle system. Features include an augmented posterior sloped talus to address subsidence by rebuilding posterior talar height, and an anatomic design that mimics natural ankle kinematics. The system received FDA 510(k) clearance in 2016 as the Salto XT; Integra had acquired U.S. rights to the system from Tornier in 2015.
For the nine months ended September 30, 2018, Integra posted Joint Recon Extremities revenue of $27.6MM, up 2.4% from the prior year, and Trauma device revenue of $43.7MM, -5.8%. The company lowered their overall organic revenue growth goal for the year, in part due to disappointing performance in their orthopaedic business. Within the segment, growth in U.S. ankle and shoulder product lines was not enough to offset a mid-teens decline in the lower extremity fixation portfolio. Leadership expects improved performance in 2019, aided by new products like Integra XT Revision and the Panta II fusion nail.
Sources: Integra LifeSciences Corp.; ORTHOWORLD Inc.

You are out of free articles for this month
Subscribe as a Guest for $0 and unlock a total of 5 articles per month.
You are out of five articles for this month
Subscribe as an Executive Member for access to unlimited articles, THE ORTHOPAEDIC INDUSTRY ANNUAL REPORT and more.
JV
Julie Vetalice is ORTHOWORLD's Editorial Assistant. She has covered the orthopedic industry for over 20 years, having joined the company in 1999.