
 Copy to clipboard
Copy to clipboard 
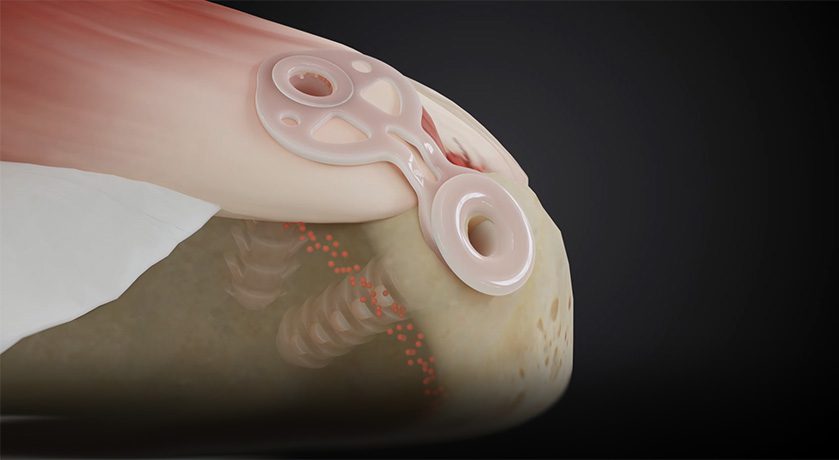
Inovedis announced that the first two patients were treated with the SINEFIX Rotator Cuff Repair System as part of a premarket clinical study in Tübingen, Germany.
Inovedis received FDA marketing clearance in 2023 and plans to enroll clinical sites into a limited U.S. market release in early 2024. The premarket clinical investigation in Europe will provide the clinical data needed in support of CE Mark submission.
“Suture anchor technology, the current clinical gold standard for rotator cuff repair surgery, has been used for decades and has been continuously optimized and diversified. Unfortunately, this approach is complex and has not shown significant improvement in patient outcomes. Often several suture anchors are stretched over the tendon to the attachment point potentially causing pressure peaks, bruising and strangulation of the tendon tissue,” according to Inventor and Co-founder of Inovedis, Stefan Welte, MD. “SINEFIX distributes shear stress uniformly to avoid punctual pressure peaks and therefore improve blood circulation and the vitality of the tendon.”
Lukas Flöss, Co-Founder and Chief Executive Officer of Inovedis, said, “Successfully beginning the treatment of patients with the SINEFIX implant is a tremendous milestone, both for our company and rotator cuff patients globally. We believe there is a need for a simplified surgical technique for rotator cuff tears designed to reduce surgical time and errors, and that our technology has the potential to facilitate efficient treatment of patients in need.”
Source: Inovedis GmbH
Inovedis announced that the first two patients were treated with the SINEFIX Rotator Cuff Repair System as part of a premarket clinical study in Tübingen, Germany.
Inovedis received FDA marketing clearance in 2023 and plans to enroll clinical sites into a limited U.S. market release in early 2024. The premarket clinical investigation in...
Inovedis announced that the first two patients were treated with the SINEFIX Rotator Cuff Repair System as part of a premarket clinical study in Tübingen, Germany.
Inovedis received FDA marketing clearance in 2023 and plans to enroll clinical sites into a limited U.S. market release in early 2024. The premarket clinical investigation in Europe will provide the clinical data needed in support of CE Mark submission.
“Suture anchor technology, the current clinical gold standard for rotator cuff repair surgery, has been used for decades and has been continuously optimized and diversified. Unfortunately, this approach is complex and has not shown significant improvement in patient outcomes. Often several suture anchors are stretched over the tendon to the attachment point potentially causing pressure peaks, bruising and strangulation of the tendon tissue,” according to Inventor and Co-founder of Inovedis, Stefan Welte, MD. “SINEFIX distributes shear stress uniformly to avoid punctual pressure peaks and therefore improve blood circulation and the vitality of the tendon.”
Lukas Flöss, Co-Founder and Chief Executive Officer of Inovedis, said, “Successfully beginning the treatment of patients with the SINEFIX implant is a tremendous milestone, both for our company and rotator cuff patients globally. We believe there is a need for a simplified surgical technique for rotator cuff tears designed to reduce surgical time and errors, and that our technology has the potential to facilitate efficient treatment of patients in need.”
Source: Inovedis GmbH

You are out of free articles for this month
Subscribe as a Guest for $0 and unlock a total of 5 articles per month.
You are out of five articles for this month
Subscribe as an Executive Member for access to unlimited articles, THE ORTHOPAEDIC INDUSTRY ANNUAL REPORT and more.
JV
Julie Vetalice is ORTHOWORLD's Editorial Assistant. She has covered the orthopedic industry for over 20 years, having joined the company in 1999.







