
 Copy to clipboard
Copy to clipboard 
First patients have been enrolled in AlloSource’s clinical study, A Randomized, Controlled Study to Evaluate Effectiveness of AlloWrap® Amniotic Membrane for the Reduction of Post-Operative Soft Tissue Inflammation in Two-Level Anterior Cervical Discectomy and Fusion (ACDF) Procedures.
The study will examine the effectiveness of AlloWrap Amniotic Membrane in the reduction of soft tissue swelling in two-level ACDF.
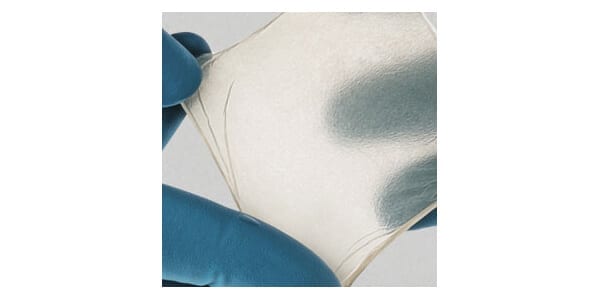
The human amniotic membrane is designed to provide a biologic barrier following surgical repair. The product is suitable for spinal procedures because, when used as a surgical barrier, the dual-sided design provides covering and protection from the surrounding environment to support the body’s natural ability to ease potential complications such as inflammation, scarring and adhesion.
AlloWrap DS has a moist, hydrated format for use in endoscopic and minimally invasive applications, whereas AlloWrap Dry is dehydrated for precision open-surgical placement.
“Previous animal studies using AlloWrap showed anti-inflammatory response,” said Dr. Ross Wilkins, AlloSource Senior Medical Director. “AlloWrap has been used in other areas of the body for years, and this clinical study will deepen our understanding of its impact in spine applications too.”
First patients have been enrolled in AlloSource's clinical study, A Randomized, Controlled Study to Evaluate Effectiveness of AlloWrap® Amniotic Membrane for the Reduction of Post-Operative Soft Tissue Inflammation in Two-Level Anterior Cervical Discectomy and Fusion (ACDF) Procedures.
The study will examine the effectiveness of AlloWrap...
First patients have been enrolled in AlloSource’s clinical study, A Randomized, Controlled Study to Evaluate Effectiveness of AlloWrap® Amniotic Membrane for the Reduction of Post-Operative Soft Tissue Inflammation in Two-Level Anterior Cervical Discectomy and Fusion (ACDF) Procedures.
The study will examine the effectiveness of AlloWrap Amniotic Membrane in the reduction of soft tissue swelling in two-level ACDF.
The human amniotic membrane is designed to provide a biologic barrier following surgical repair. The product is suitable for spinal procedures because, when used as a surgical barrier, the dual-sided design provides covering and protection from the surrounding environment to support the body’s natural ability to ease potential complications such as inflammation, scarring and adhesion.
AlloWrap DS has a moist, hydrated format for use in endoscopic and minimally invasive applications, whereas AlloWrap Dry is dehydrated for precision open-surgical placement.
“Previous animal studies using AlloWrap showed anti-inflammatory response,” said Dr. Ross Wilkins, AlloSource Senior Medical Director. “AlloWrap has been used in other areas of the body for years, and this clinical study will deepen our understanding of its impact in spine applications too.”

You are out of free articles for this month
Subscribe as a Guest for $0 and unlock a total of 5 articles per month.
You are out of five articles for this month
Subscribe as an Executive Member for access to unlimited articles, THE ORTHOPAEDIC INDUSTRY ANNUAL REPORT and more.
JV
Julie Vetalice is ORTHOWORLD's Editorial Assistant. She has covered the orthopedic industry for over 20 years, having joined the company in 1999.







