
 Copy to clipboard
Copy to clipboard 
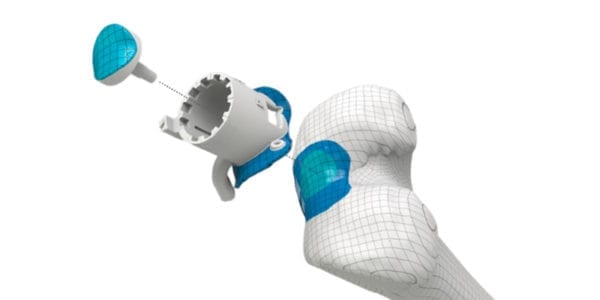
Episurf Medical announced the first Episealer® surgery in the U.S. The surgery was performed within the company’s Investigational Device Exemption (IDE) clinical trial, EPIC-Knee: Episealer Knee System IDE Clinical Study.
“This is, of course, a great milestone for us, and we would like to extend our gratitude to everyone involved. Many more surgeries are to come in the US, but the first surgery in the US is a special milestone for us,” said Pål Ryfors, CEO of Episurf Medical.
Episurf Medical announced the first Episealer® surgery in the U.S. The surgery was performed within the company's Investigational Device Exemption (IDE) clinical trial, EPIC-Knee: Episealer Knee System IDE Clinical Study.
"This is, of course, a great milestone for us, and we would like to extend our...
Episurf Medical announced the first Episealer® surgery in the U.S. The surgery was performed within the company’s Investigational Device Exemption (IDE) clinical trial, EPIC-Knee: Episealer Knee System IDE Clinical Study.
“This is, of course, a great milestone for us, and we would like to extend our gratitude to everyone involved. Many more surgeries are to come in the US, but the first surgery in the US is a special milestone for us,” said Pål Ryfors, CEO of Episurf Medical.

You are out of free articles for this month
Subscribe as a Guest for $0 and unlock a total of 5 articles per month.
You are out of five articles for this month
Subscribe as an Executive Member for access to unlimited articles, THE ORTHOPAEDIC INDUSTRY ANNUAL REPORT and more.
JV
Julie Vetalice is ORTHOWORLD's Editorial Assistant. She has covered the orthopedic industry for over 20 years, having joined the company in 1999.







