
 Copy to clipboard
Copy to clipboard 
Episurf Medical announced the scheduling of the first surgery with the Episealer® knee implant in the Asia-Pacific region. This is the first procedure outside of Europe.
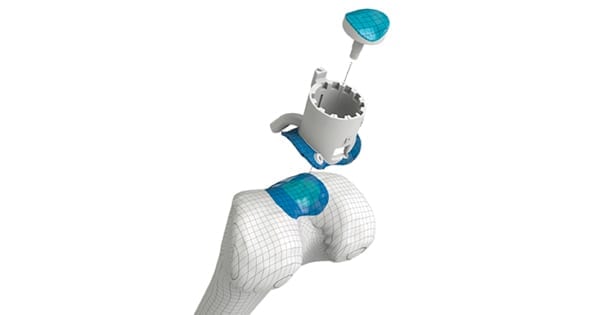
Episealer is available for the knee femoral condyles (medial and lateral), the knee trochlea area or both. Episealer and its instrument set are designed to remove only the damaged tissue as well as restore the area with a patient-specific implant. Using an individualized drill guide during surgery is intended to increase the precision of the procedure for optimal positioning of the implant.
“This is a significant milestone for us, and we have identified significant opportunities in the Asia-Pacific region. Recently, we have increased our focus towards several new markets, in which the regulatory work is ongoing. Scheduling the first surgery outside of Europe is exciting. We are also happy for the fact that we are reaching commercial milestones, simultaneously as we are making significant progress within the clinical strategy,” said Pål Ryfors, CEO.
Episurf Medical announced the scheduling of the first surgery with the Episealer® knee implant in the Asia-Pacific region. This is the first procedure outside of Europe.
Episealer is available for the knee femoral condyles (medial and lateral), the knee trochlea area or both. Episealer and its instrument set are designed to remove only the...
Episurf Medical announced the scheduling of the first surgery with the Episealer® knee implant in the Asia-Pacific region. This is the first procedure outside of Europe.
Episealer is available for the knee femoral condyles (medial and lateral), the knee trochlea area or both. Episealer and its instrument set are designed to remove only the damaged tissue as well as restore the area with a patient-specific implant. Using an individualized drill guide during surgery is intended to increase the precision of the procedure for optimal positioning of the implant.
“This is a significant milestone for us, and we have identified significant opportunities in the Asia-Pacific region. Recently, we have increased our focus towards several new markets, in which the regulatory work is ongoing. Scheduling the first surgery outside of Europe is exciting. We are also happy for the fact that we are reaching commercial milestones, simultaneously as we are making significant progress within the clinical strategy,” said Pål Ryfors, CEO.

You’ve reached your limit.
We’re glad you’re finding value in our content — and we’d love for you to keep going.
Subscribe now for unlimited access to orthopedic business intelligence.
JV
Julie Vetalice is ORTHOWORLD's Editorial Assistant. She has covered the orthopedic industry for over 20 years, having joined the company in 1999.







