
 Copy to clipboard
Copy to clipboard 
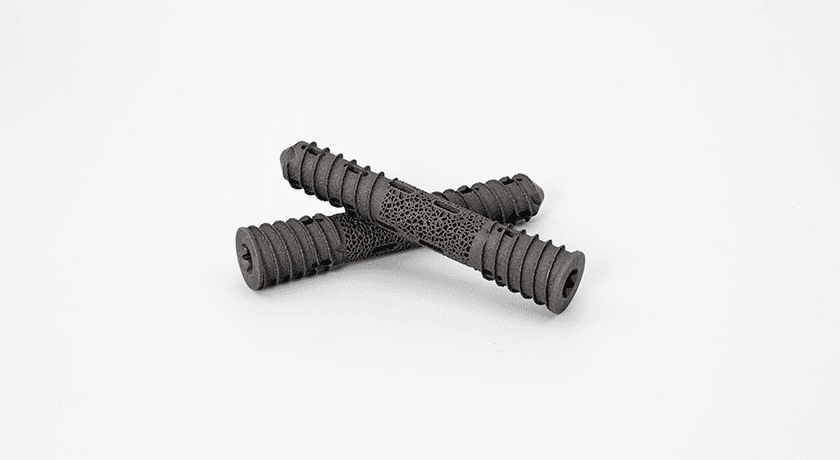
OrthoFundamentals announced successful first cases of the Trellis™ SI Joint Fusion System from both a direct lateral and a posterolateral oblique approach. The Trellis platform comprises sterile, single-use disposable instrument kits paired with 3D-printed implants.
With the OrthoFundamentals solution, instruments and implants are provided to the facilities in the OrthoPod™ smart storage system which reads RFID tags on all packages. This allows OrthoFundamentals and all assigned users the ability to track their inventory in real-time and be alerted to expiring product and par levels. It also allows for lot traceability, from the raw powder of the additively manufactured implants along its journey into the patient.
When asked about the design rationale, the company’s CEO Matthew Palmer stated, “Safety, efficiency, and price-points were at the center of the design process for the system. With implant technology becoming increasingly commoditized, we felt that we had an opportunity to bring a better solution to market that addressed the needs of the customer in outpatient centers by changing the business model instead of iterating on implant features. Traditional medical device firms are simply not meeting the needs of the outpatient centers with their bulky, reusable instrument sets and repeatedly reprocessed implants which get driven from place to place by sales reps. We provide surgery-ready solutions that come pre-sterilized and can be recycled at the end of the case.”
Trellis SI Joint Fusion was cleared for marketing by FDA in April 2022.
Source: OrthoFundamentals
OrthoFundamentals announced successful first cases of the Trellis™ SI Joint Fusion System from both a direct lateral and a posterolateral oblique approach. The Trellis platform comprises sterile, single-use disposable instrument kits paired with 3D-printed implants.
With the OrthoFundamentals solution, instruments and implants are provided to...
OrthoFundamentals announced successful first cases of the Trellis™ SI Joint Fusion System from both a direct lateral and a posterolateral oblique approach. The Trellis platform comprises sterile, single-use disposable instrument kits paired with 3D-printed implants.
With the OrthoFundamentals solution, instruments and implants are provided to the facilities in the OrthoPod™ smart storage system which reads RFID tags on all packages. This allows OrthoFundamentals and all assigned users the ability to track their inventory in real-time and be alerted to expiring product and par levels. It also allows for lot traceability, from the raw powder of the additively manufactured implants along its journey into the patient.
When asked about the design rationale, the company’s CEO Matthew Palmer stated, “Safety, efficiency, and price-points were at the center of the design process for the system. With implant technology becoming increasingly commoditized, we felt that we had an opportunity to bring a better solution to market that addressed the needs of the customer in outpatient centers by changing the business model instead of iterating on implant features. Traditional medical device firms are simply not meeting the needs of the outpatient centers with their bulky, reusable instrument sets and repeatedly reprocessed implants which get driven from place to place by sales reps. We provide surgery-ready solutions that come pre-sterilized and can be recycled at the end of the case.”
Trellis SI Joint Fusion was cleared for marketing by FDA in April 2022.
Source: OrthoFundamentals

You are out of free articles for this month
Subscribe as a Guest for $0 and unlock a total of 5 articles per month.
You are out of five articles for this month
Subscribe as an Executive Member for access to unlimited articles, THE ORTHOPAEDIC INDUSTRY ANNUAL REPORT and more.
JV
Julie Vetalice is ORTHOWORLD's Editorial Assistant. She has covered the orthopedic industry for over 20 years, having joined the company in 1999.







