

 Copy to clipboard
Copy to clipboard 
TheraCell completed the first surgical case using its TheraFuze DBF® Fiber Wrap™. DBF Fiber Wrap is offered as a lyophilized sheet of 100% demineralized cortical bone fibers that can be rehydrated with saline or the patient’s blood or bone marrow to create a strong, flexible sheet.
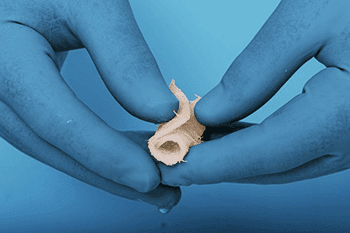
The Fiber Wrap is designed to maintain form and integrity when rehydrated and to withstand manipulation by the surgeon.
TheraFuze DBF Fiber Wraps are made from cortical demineralized bone fibers and processed using proprietary Bone Textile™ technology. The processes were developed to produce uniform fiber geometry, size and texture. FiberLok™ technology allows Fiber Wrap products to maintain their form and integrity after rehydration, permitting adequate time for manipulation and implantation.
“We are pleased to have achieved our 2020 product launch objectives for the TheraFuze DBF product line, and we look forward to commercializing a few additional products in the first half of 2021,” said Andy Carter, Ph.D., TheraCell’s Chief Technology Officer.
TheraCell completed the first surgical case using its TheraFuze DBF® Fiber Wrap™. DBF Fiber Wrap is offered as a lyophilized sheet of 100% demineralized cortical bone fibers that can be rehydrated with saline or the patient's blood or bone marrow to create a strong, flexible sheet.
TheraFuze DBF Fiber Wraps are made from cortical...
TheraCell completed the first surgical case using its TheraFuze DBF® Fiber Wrap™. DBF Fiber Wrap is offered as a lyophilized sheet of 100% demineralized cortical bone fibers that can be rehydrated with saline or the patient’s blood or bone marrow to create a strong, flexible sheet.

The Fiber Wrap is designed to maintain form and integrity when rehydrated and to withstand manipulation by the surgeon.
TheraFuze DBF Fiber Wraps are made from cortical demineralized bone fibers and processed using proprietary Bone Textile™ technology. The processes were developed to produce uniform fiber geometry, size and texture. FiberLok™ technology allows Fiber Wrap products to maintain their form and integrity after rehydration, permitting adequate time for manipulation and implantation.
“We are pleased to have achieved our 2020 product launch objectives for the TheraFuze DBF product line, and we look forward to commercializing a few additional products in the first half of 2021,” said Andy Carter, Ph.D., TheraCell’s Chief Technology Officer.

You are out of free articles for this month
Subscribe as a Guest for $0 and unlock a total of 5 articles per month.
You are out of five articles for this month
Subscribe as an Executive Member for access to unlimited articles, THE ORTHOPAEDIC INDUSTRY ANNUAL REPORT and more.
JV
Julie Vetalice is ORTHOWORLD's Editorial Assistant. She has covered the orthopedic industry for over 20 years, having joined the company in 1999.







