
 Copy to clipboard
Copy to clipboard 
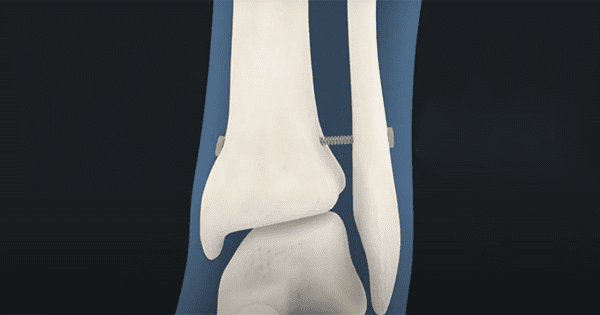
Panther Orthopedics’ PUMA System™ has been successfully used in Lapidus surgeries. The PUMA System is the first and only FDA-cleared superelastic fixation device that maintains continuous compression without creep. Panther’s soft tissue platform technology offers biomechanical and clinical advantages because its spring-like design moves with the body while still maintaining continuous fixation.
PUMA is a nitinol-based soft tissue fixation device that uses cyclical loading to provide stabilization without over-compression or loosening. Bringing nitinol into use for soft tissue applications with the PUMA System is a novel innovation, providing significant advantages over existing screw or endobutton type fixation devices. Superelastic stabilization provides creep-free, continuous compression, reducing over-compression and subsequent adverse effects that impact patient quality of life. Nitinol has a proven safety record in foot and ankle surgical repair and reconstruction implants.
Previous clinical assessments have demonstrated the PUMA System’s favorable profile for effectively treating ankle syndesmosis injuries. In 2020, a mid-term clinical assessment studying the use of the PUMA System in patients with sydesmosis disruptions followed over 50 patients over 22 months, tracking procedural safety and healing response via postoperative radiographic and clinical evaluations. The assessment confirmed satisfactory radiographic syndesmosis healing with reduction in the ankle mortise in 100% of patients, finding no evidence of lysis, device migration, sydesmotic widening, infections, soft tissue complications, or need for revision surgeries.
Source: Panther Orthopedics
Panther Orthopedics' PUMA System™ has been successfully used in Lapidus surgeries. The PUMA System is the first and only FDA-cleared superelastic fixation device that maintains continuous compression without creep. Panther's soft tissue platform technology offers biomechanical and clinical advantages because its spring-like design moves with the...
Panther Orthopedics’ PUMA System™ has been successfully used in Lapidus surgeries. The PUMA System is the first and only FDA-cleared superelastic fixation device that maintains continuous compression without creep. Panther’s soft tissue platform technology offers biomechanical and clinical advantages because its spring-like design moves with the body while still maintaining continuous fixation.
PUMA is a nitinol-based soft tissue fixation device that uses cyclical loading to provide stabilization without over-compression or loosening. Bringing nitinol into use for soft tissue applications with the PUMA System is a novel innovation, providing significant advantages over existing screw or endobutton type fixation devices. Superelastic stabilization provides creep-free, continuous compression, reducing over-compression and subsequent adverse effects that impact patient quality of life. Nitinol has a proven safety record in foot and ankle surgical repair and reconstruction implants.
Previous clinical assessments have demonstrated the PUMA System’s favorable profile for effectively treating ankle syndesmosis injuries. In 2020, a mid-term clinical assessment studying the use of the PUMA System in patients with sydesmosis disruptions followed over 50 patients over 22 months, tracking procedural safety and healing response via postoperative radiographic and clinical evaluations. The assessment confirmed satisfactory radiographic syndesmosis healing with reduction in the ankle mortise in 100% of patients, finding no evidence of lysis, device migration, sydesmotic widening, infections, soft tissue complications, or need for revision surgeries.
Source: Panther Orthopedics

You are out of free articles for this month
Subscribe as a Guest for $0 and unlock a total of 5 articles per month.
You are out of five articles for this month
Subscribe as an Executive Member for access to unlimited articles, THE ORTHOPAEDIC INDUSTRY ANNUAL REPORT and more.
JV
Julie Vetalice is ORTHOWORLD's Editorial Assistant. She has covered the orthopedic industry for over 20 years, having joined the company in 1999.







